Abstract
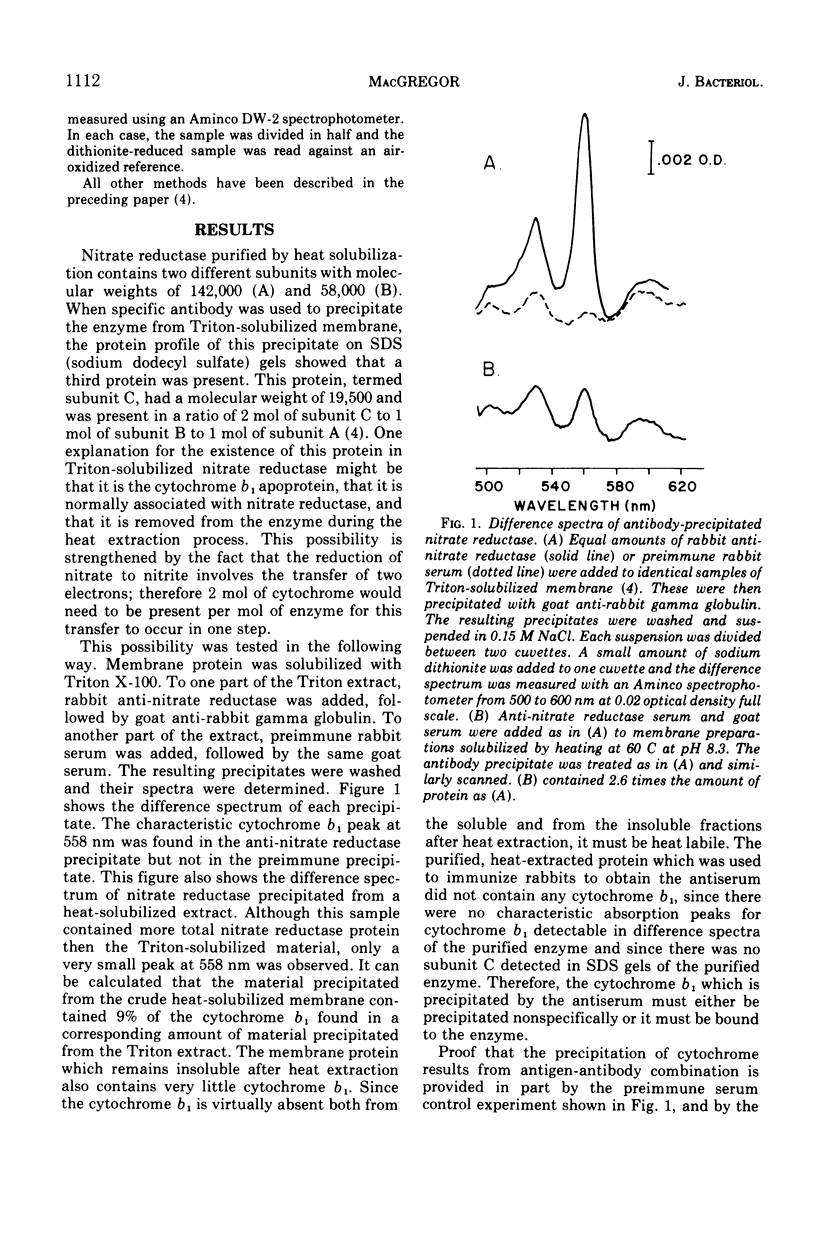
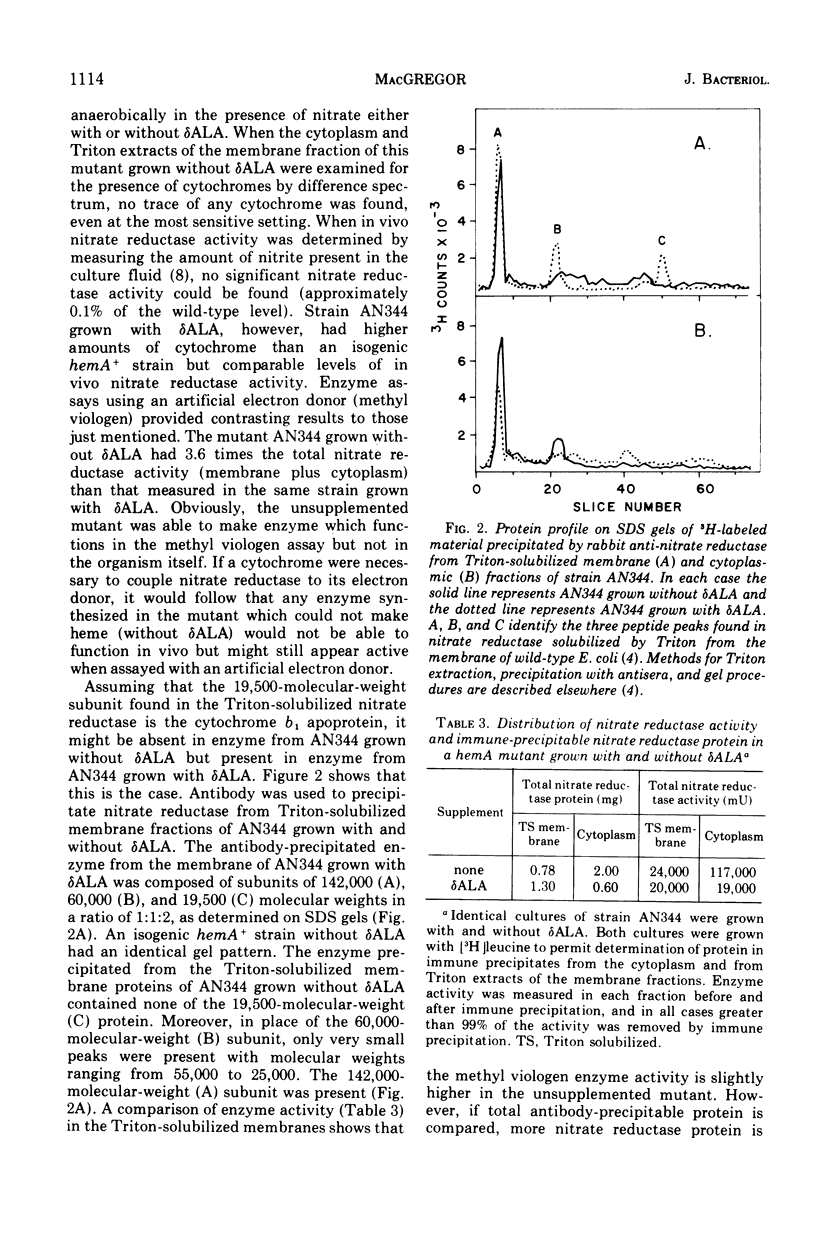
Nitrate reductase solubilized from the membrane of Escherichia coli by alkaline heat treatment was purified to homogeneity and used to prepare specific antibody. Nitrate reductase, precipitated by this antibody from Triton extracts of the membrane, contained a third subunit, not present in the purified enzyme used to prepare the antibody. This third subunit was identified as the cytochrome b1 apoprotein. This cytochrome is bound to nitrate reductase from wild-type E. coli in a ratio of 2 mol of cytochrome per mol of enzyme complex. In mutants unable to synthesize heme, this cytochrome b1 apoprotein is not bound to nitrate reductase. In these same mutants, the enzyme is overproduced and accumulates in the cytoplasm. The absence of cytochrome also affects the stability of the membrane-bound form of the enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Haddock B. A., Schairer H. U. Electron-transport chains of Escherichia coli. Reconstitution of respiration in a 5-aminolaevulinic acid-requiring mutant. Eur J Biochem. 1973 May;35(1):34–45. doi: 10.1111/j.1432-1033.1973.tb02806.x. [DOI] [PubMed] [Google Scholar]
- LINNANE A. W., WRIGLEY C. W. FRAGMENTATION OF THE ELECTRON TRANSPORT CHAIN OF ESCHERICHIA COLI. PREPARATION OF A SOLUBLE FORMATE DEHYDROGENASE-CYTOCHROME B1 COMPLEX. Biochim Biophys Acta. 1963 Nov 8;77:408–418. doi: 10.1016/0006-3002(63)90515-8. [DOI] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A. Alterations in the cytoplasmic membrane proteins of various chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1971 Oct;108(1):564–570. doi: 10.1128/jb.108.1.564-570.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A., Normansell D. E. Purification and properties of nitrate reductase from Escherichia coli K12. J Biol Chem. 1974 Aug 25;249(16):5321–5327. [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A. Reconstitution of nitrate reductase activity and formation of membrane particles from cytoplasmic extracts of chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1164–1176. doi: 10.1128/jb.114.3.1164-1176.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H. Solubilization of Escherichia coli nitrate reductase by a membrane-bound protease. J Bacteriol. 1975 Mar;121(3):1102–1110. doi: 10.1128/jb.121.3.1102-1110.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H. Synthesis of nitrate reductase components in chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1975 Mar;121(3):1117–1121. doi: 10.1128/jb.121.3.1117-1121.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Herrera J., DeMoss J. A. Nitrate reductase complex of Escherichia coli K-12: participation of specific formate dehydrogenase and cytochrome b1 components in nitrate reduction. J Bacteriol. 1969 Sep;99(3):720–729. doi: 10.1128/jb.99.3.720-729.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANIGUCHI S., ITAGAKI E. Nitrate reductase of nitrate respiration type from E. coli. I. Solubilization and purification from the particulate system with molecular characterization as a metalloprotein. Biochim Biophys Acta. 1960 Nov 4;44:263–279. doi: 10.1016/0006-3002(60)91562-6. [DOI] [PubMed] [Google Scholar]


