Abstract
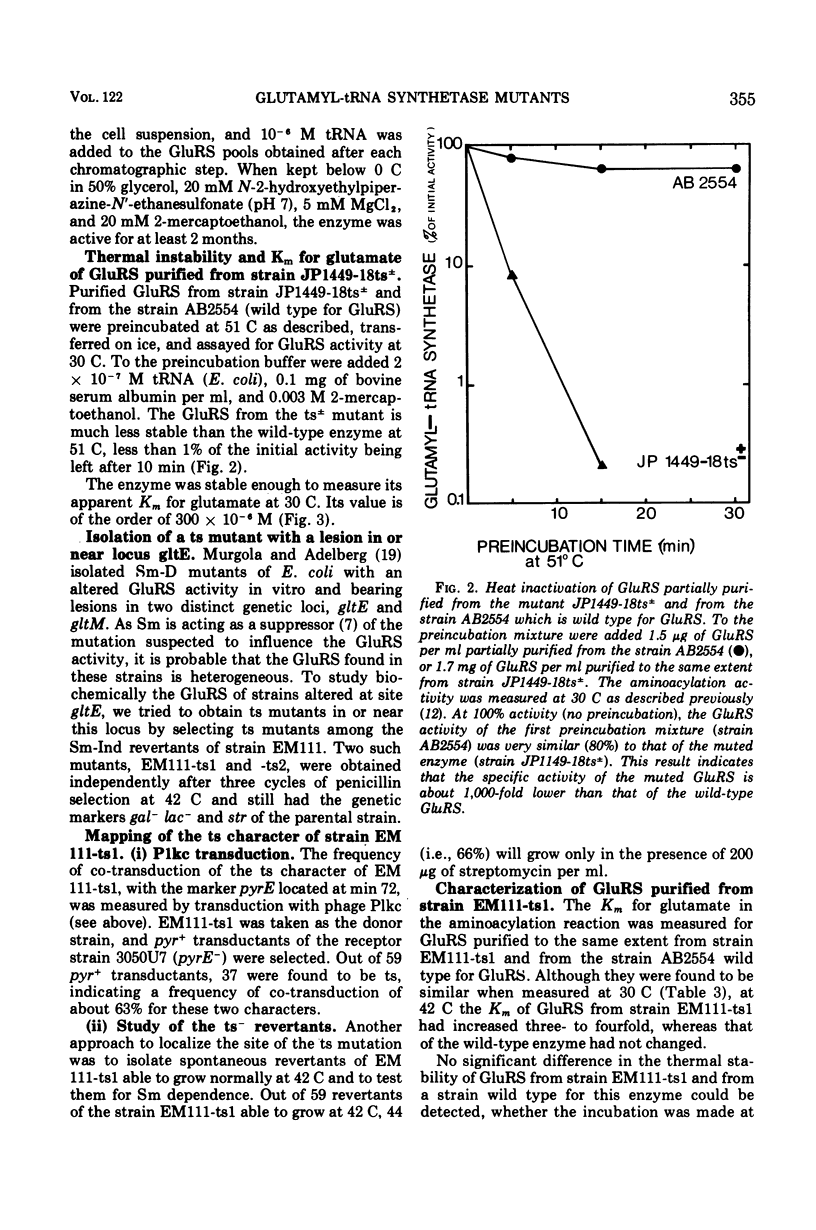
The glutamyl-transfer ribonucleic acid synthetase (GluRS) of a partial revertants (ts plus or minus) of the thermosensitive (ts) mutant strain JP1449 (LOcus gltx) and of a ts mutant strain EM111-ts1 with a lesion in or near the locus gltx have been studied to find the relation between these two genetic loci known to influence the GluRS activity in vitro and the presence of a catalytic subunit and of a regulatory subunit in the GluRS purified from Escherichia coli K-12. The ts character of strain JP1449-18ts plus or minus is co-transduced with the marker dsdA at the same frequency as is the ts character of strain JP1449. Its purified GluRS is very thermolabile and its Km for glutamate is higher than that of a wild-type GluRS. These results indicate that the locus gltX is in the structural gene for the catalytic subunit of this enzyme. The location of the mutation causing the partial ts reversion in strain JP1449-18ts plus or minus is discussed. The GluRS purified from the ts mutant strain EM111-ts1 has the same stability as the wild-type enzyme, but its Km forglutamate increases with the temperature, suggesting that the locus gltE codes for a regulatory factor, possibly for the polypeptide chain that is co-purified with the catalytic subunit.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckel P., Ruffler D., Piepersberg W., Böck A. RNA overproducing revertants of an alanyl-tRNA synthetase mutant of Escherichia coli. Mol Gen Genet. 1972;119(4):323–335. doi: 10.1007/BF00272090. [DOI] [PubMed] [Google Scholar]
- Cassio D., Waller J. P. Modification of methionyl-tRNA synthetase by proteolytic cleavage and properties of the trypsin-modified enzyme. Eur J Biochem. 1971 May 28;20(2):283–300. doi: 10.1111/j.1432-1033.1971.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Clarke S. J., Low B., Konigsberg W. Isolation and characterization of a regulatory mutant of an aminoacyl-transfer ribonucleic acid synthetase in Escherichia coli K-12. J Bacteriol. 1973 Mar;113(3):1096–1103. doi: 10.1128/jb.113.3.1096-1103.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANGMAN W. L., NEIDHARDT F. C. DEMONSTRATION OF AN ALTERED AMINOACYL RIBONUCLEIC ACID SYNTHETASE IN A MUTANT OF ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1839–1843. [PubMed] [Google Scholar]
- Folk W. R., Berg P. Characterization of altered forms of glycyl transfer ribonucleic acid synthetase and the effects of such alterations on aminoacyl transfer ribonucleic acid synthesis in vivo. J Bacteriol. 1970 Apr;102(1):204–212. doi: 10.1128/jb.102.1.204-212.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., KATAJA E. PHENOTYPIC REPAIR BY STREPTOMYCIN OF DEFECTIVE GENOTYPES IN E. COLI. Proc Natl Acad Sci U S A. 1964 Mar;51:487–493. doi: 10.1073/pnas.51.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N., Sinsheimer R. L. Lysis of Escherichia coli with a neutral detergent. Biochim Biophys Acta. 1967 Dec 19;149(2):476–488. doi: 10.1016/0005-2787(67)90175-x. [DOI] [PubMed] [Google Scholar]
- Kan J., Sueoka N. Further evidence for a single leucyl transfer ribonucleic acid synthetase capable of charging five leucine transfer ribonucleic acids in Escherichia coli. J Biol Chem. 1971 Apr 10;246(7):2207–2210. [PubMed] [Google Scholar]
- Kaplan S., Anderson D. Selection of temperature-sensitive activating enzyme mutants in Escherichia coli. J Bacteriol. 1968 Mar;95(3):991–997. doi: 10.1128/jb.95.3.991-997.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisselev L. L., Baturina I. D. Two enzymatically active forms of lysyl-tRNA synthetase from E. coli B. FEBS Lett. 1972 May 1;22(2):231–234. doi: 10.1016/0014-5793(72)80052-8. [DOI] [PubMed] [Google Scholar]
- Kisselev L. L., Favorova O. O. Aminoacyl-tRNA synthetases: sone recent results and achievements. Adv Enzymol Relat Areas Mol Biol. 1974;40(0):141–238. doi: 10.1002/9780470122853.ch5. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lapointe J., Söll D. Glutamyl transfer ribonucleic acid synthetase of Escherichia coli. 3. Influence of the 46K protein on the affinity of the 56K glutamyl transfer ribonucleic acid synthetase for its substrates. J Biol Chem. 1972 Aug 25;247(16):4982–4985. [PubMed] [Google Scholar]
- Lapointe J., Söll D. Glutamyl transfer ribonucleic acid synthetase of Escherichia coli. I. Purification and properties. J Biol Chem. 1972 Aug 25;247(16):4966–4974. [PubMed] [Google Scholar]
- Lupker J. H., Verschoor G. H., Glickman B. W., Rörsch A., Bosch L. Thermosensitive mutants of Escherichia coli unable to propagate RNA phage at 42 degrees C and altered in protein synthesis. Eur J Biochem. 1974 Apr 16;43(3):583–590. doi: 10.1111/j.1432-1033.1974.tb03445.x. [DOI] [PubMed] [Google Scholar]
- McFall E. Mapping of the d-serine deaminase region in Escherichia coli K-12. Genetics. 1967 Jan;55(1):91–99. doi: 10.1093/genetics/55.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgola E. J., Adelberg E. A. Mutants of Escherichia coli K-12 with an altered glutamyl-transfer ribonucleic acid synthetase. J Bacteriol. 1970 Jul;103(1):178–183. doi: 10.1128/jb.103.1.178-183.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouget P., Chapeville F. Leucyl-tRNA synthetase. Two forms of the enzyme: relation between structural and catalytic properties. Eur J Biochem. 1971 Dec 10;23(3):459–467. doi: 10.1111/j.1432-1033.1971.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Russell R. R., Pittard A. J. Mutants of Escherichia coli unable to make protein at 42 C. J Bacteriol. 1971 Nov;108(2):790–798. doi: 10.1128/jb.108.2.790-798.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R. Temperature-sensitive osmotic remedial mutants of Escherichia coli. J Bacteriol. 1972 Nov;112(2):661–665. doi: 10.1128/jb.112.2.661-665.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yem D. W., Williams L. S. Evidence for the existence of two arginyl-transfer ribonucleic acid synthetase activities in Escherichia coli. J Bacteriol. 1973 Feb;113(2):891–894. doi: 10.1128/jb.113.2.891-894.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


