Abstract
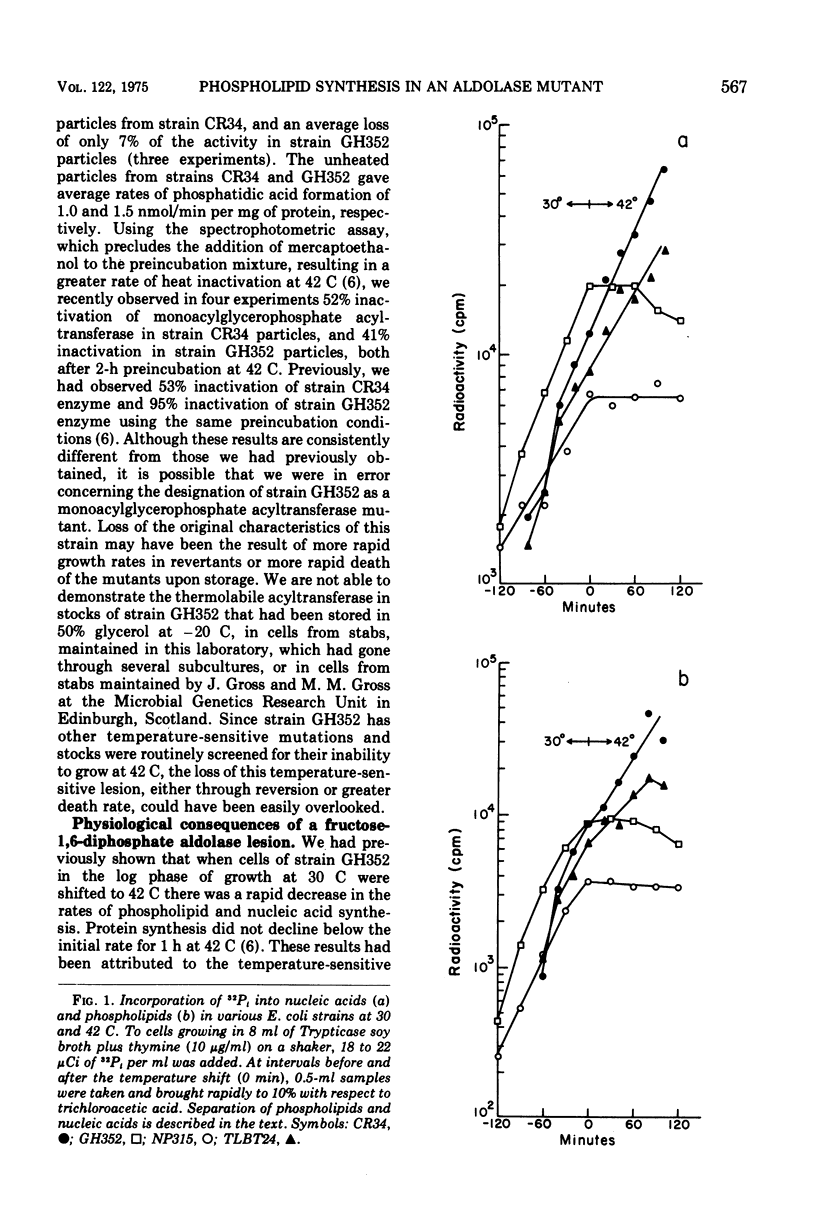
Escherichia coli GH352, which was originally described as a temperature-sensitive strain containing a thermolabile acyl coenzyme A:monoacylglycerol 3-phosphate acyltransferase, does not now contain a thermolabile form of this enzyme. It has a defect in fructose-1,6-diphosphate aldolase and at least one additional temperature-sensitive lesion. Both strains GH352 and NP315, a temperature-sensitive aldolase mutant, show rapid cessation of 32-P1 incorporation into nucleic acids and phospholipids at 42 C. These characteristics of strain GH352 are therefore no longer attributed to thermolabile phospholipid synthesis, but can be attributed to the fructose-1,6-diphophate aldolase lesion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böck A., Neidhardt F. C. Isolation of a Mutant of Escherichia coli with a Temperature-sensitive Fructose-1,6-Diphosphate Aldolase Activity. J Bacteriol. 1966 Aug;92(2):464–469. doi: 10.1128/jb.92.2.464-469.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böck A., Neidhardt F. C. Properties of a Mutant of Escherichia coli with a Temperature-sensitive Fructose-1,6-Diphosphate Aldolase. J Bacteriol. 1966 Aug;92(2):470–476. doi: 10.1128/jb.92.2.470-476.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUNCE A. L., BARNETT S. R., BEYER G. T. Further studies on the kinetics and determination of aldolase. J Biol Chem. 1950 Aug;185(2):769–780. [PubMed] [Google Scholar]
- Goldfine H., Ailhaud G. P., Vagelos P. R. Involvement of acyl carrier protein in acylation of glycerol 3-phosphate in Clostridium butyricum. II. Evidence for the participation of acyl thioesters of acyl carrier protein. J Biol Chem. 1967 Oct 10;242(19):4466–4475. [PubMed] [Google Scholar]
- Hechemy K., Goldfine H. Isolation and characterization of a temperature-sensitive mutant of Escherichia coli with a lesion in the acylation of lysophosphatidic acid. Biochem Biophys Res Commun. 1971 Jan 22;42(2):245–251. doi: 10.1016/0006-291x(71)90094-5. [DOI] [PubMed] [Google Scholar]
- Long C., Odavić R., Sargent E. J. The chemical nature of the products obtained by the action of cabbage-leaf phospholipase D on lysolecithin: the structure of lysolecithin. Biochem J. 1967 Jan;102(1):221–229. doi: 10.1042/bj1020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffler D., Böck A. Location of the structural gene for fructose-1,6-diphosphate aldolase in Escherichia coli. J Bacteriol. 1973 Nov;116(2):1054–1055. doi: 10.1128/jb.116.2.1054-1055.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M. Temperature control of phospholipid biosynthesis in Escherichia coli. J Bacteriol. 1971 May;106(2):449–455. doi: 10.1128/jb.106.2.449-455.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stribling D., Perham R. N. Purification and characterization of two fructose diphosphate aldolases from Escherichia coli (Crookes' strain). Biochem J. 1973 Apr;131(4):833–841. doi: 10.1042/bj1310833. [DOI] [PMC free article] [PubMed] [Google Scholar]


