Abstract
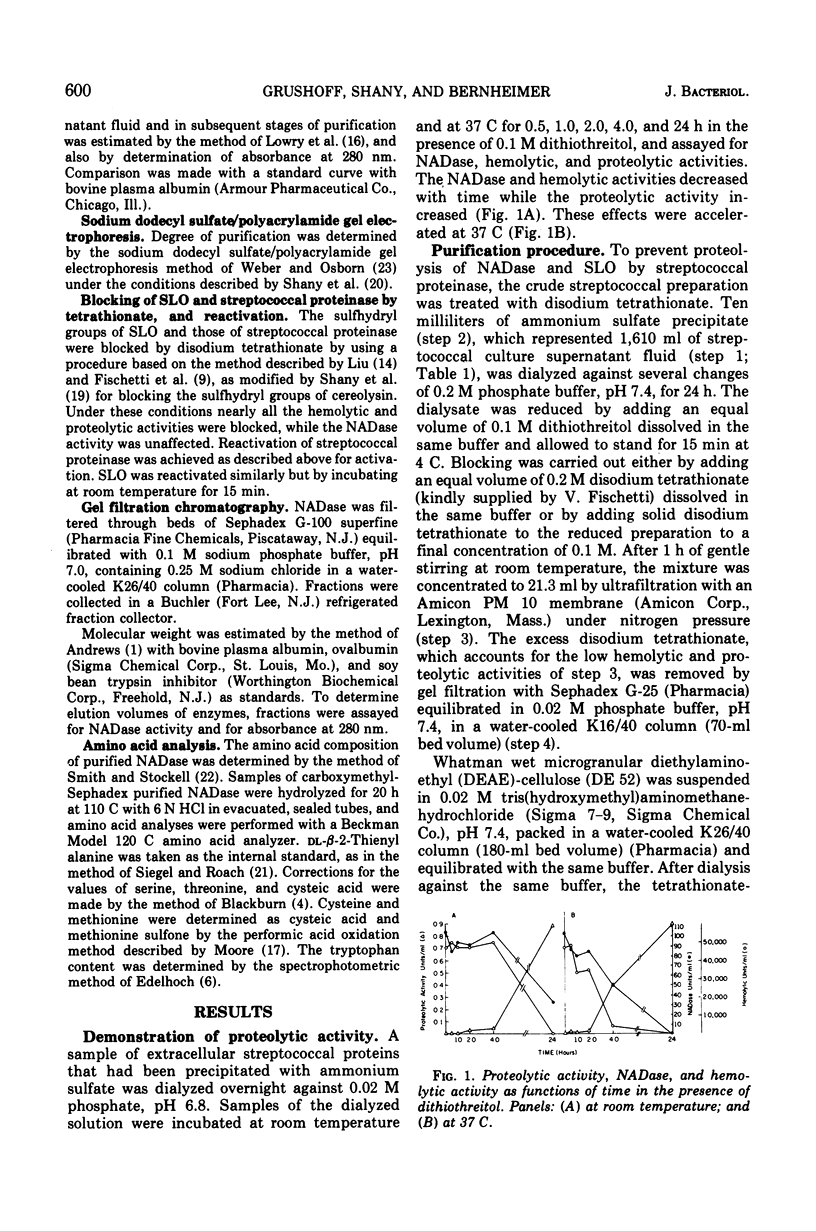
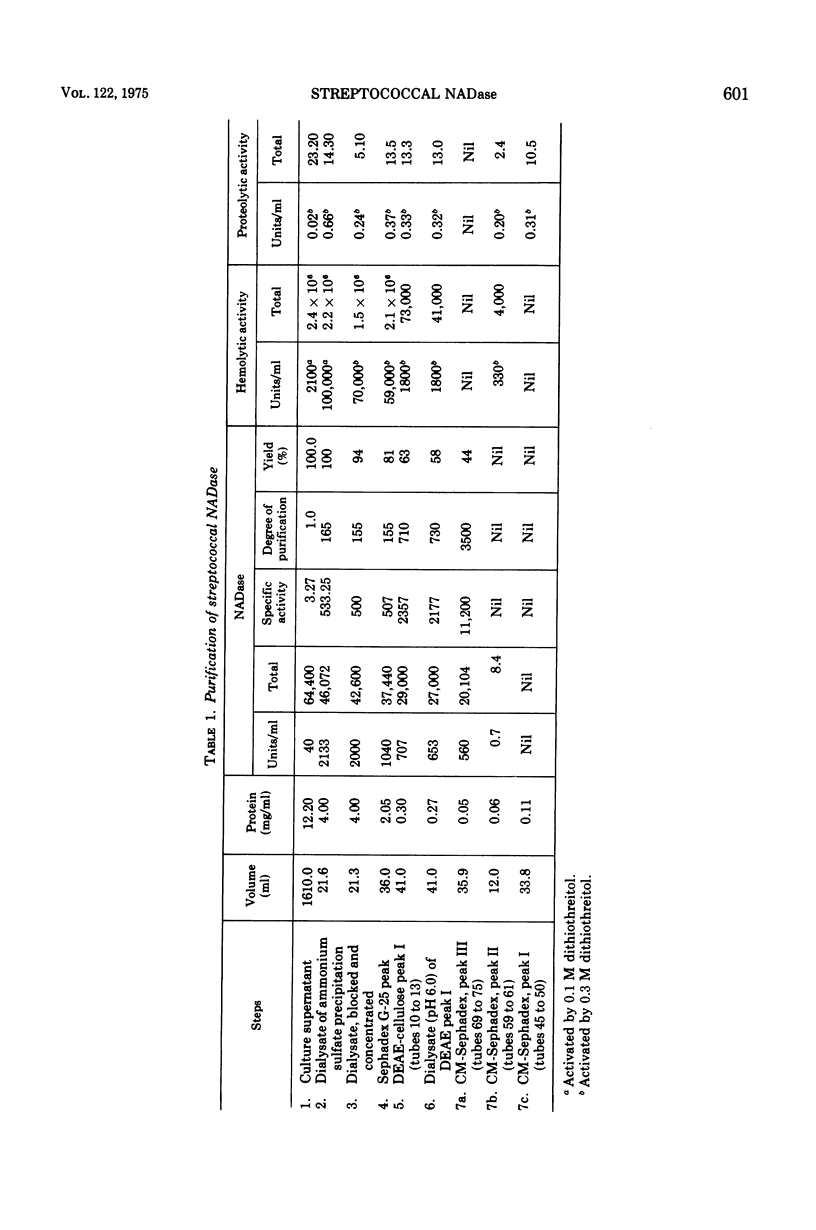
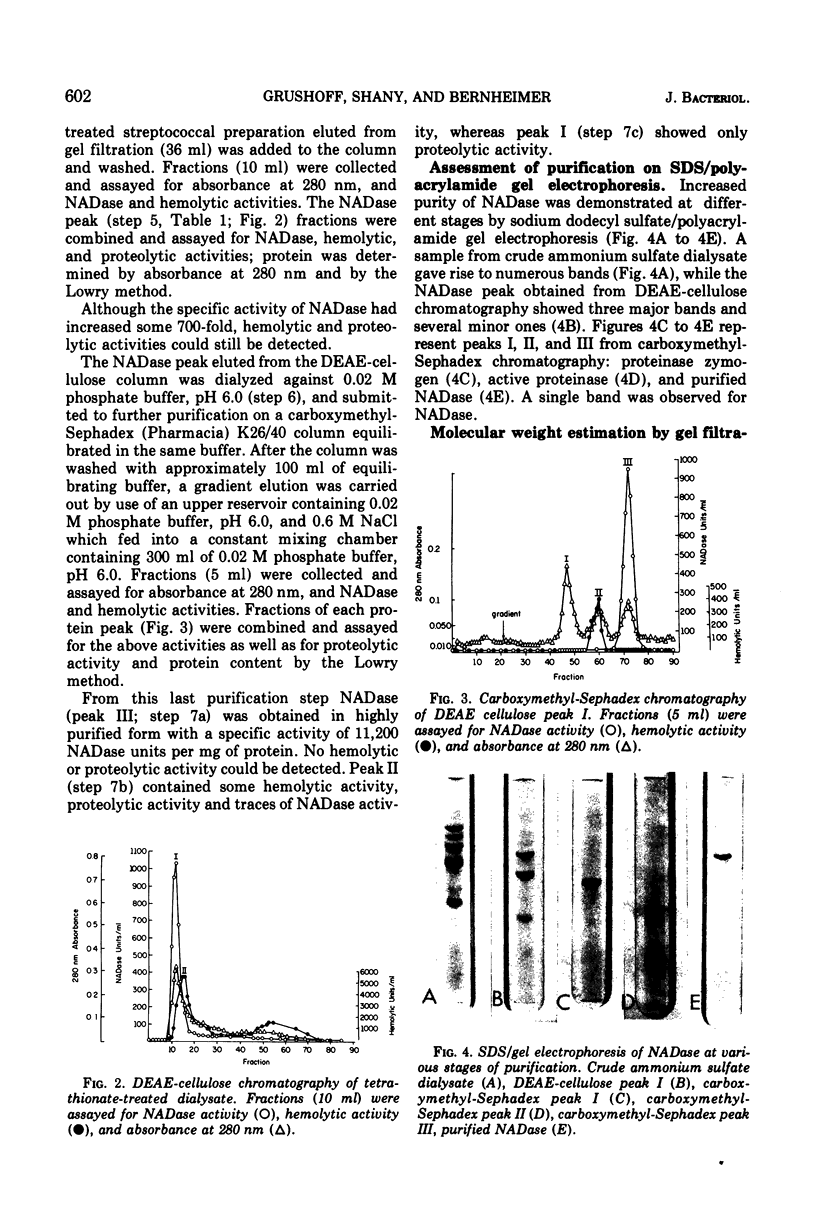
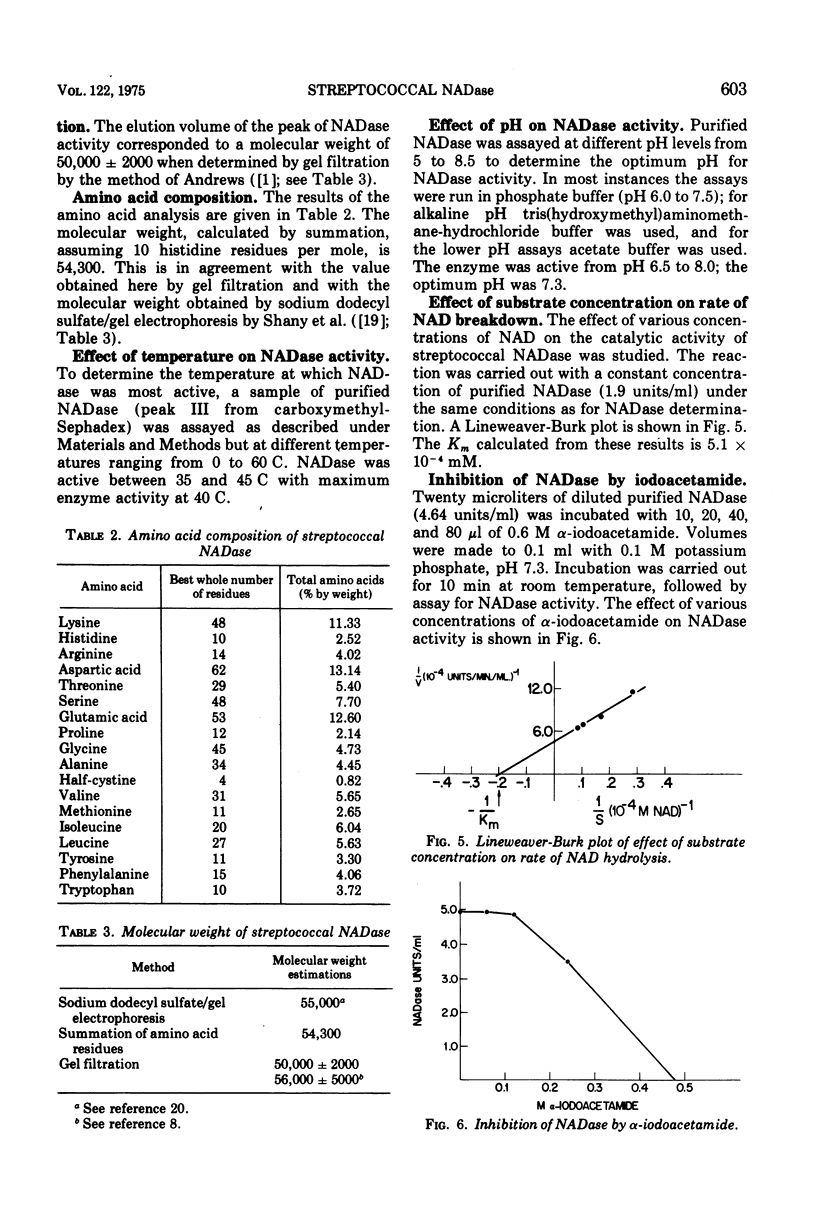
Highly purified streptococcal nicotinamide adenine dinucleotide glycohydrolase (NADase) was obtained by utilizing disodium tetrathionate to protect the enzyme by blocking the sulfhydryl groups of streptococcal proteinase. This was followed by two-step ion-exchange chromatography. The pure enzyme, demonstrated as a single band on sodium dodecyl sulfate/polyacrylamide gel electrophoresis, had a specific activity of 11,200 NADase units per mg of protein and was devoid of hemolytic activity. NADase had a molecular weight of about 55,000 as determined by gel filtration, by summation of amino acid residues, and by sodium dodecyl sulfate/gel electrophoresis. The purified enzyme had optimal activity at pH 7.3 and at 40 C and a calculated Km of 5.1 times 10- minus 4 mM. It was inhibited by alpha-iodoacetamide.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNHEIMER A. W., SCHWARTZ L. L. Isolation and composition of staphylococcal alpha toxin. J Gen Microbiol. 1963 Mar;30:455–468. doi: 10.1099/00221287-30-3-455. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W., Grushoff P. Cereolysin: production, purification and partial characterization. J Gen Microbiol. 1967 Jan;46(1):143–150. doi: 10.1099/00221287-46-1-143. [DOI] [PubMed] [Google Scholar]
- CARLSON A. S., KELLNER A., BERNHEIMER A. W., FREEMAN E. B. A streptococcal enzyme that acts specifically upon diphosphopyridine nucleotide: characterization of the enzyme and its separation from streptolysin O. J Exp Med. 1957 Jul 1;106(1):15–26. doi: 10.1084/jem.106.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Fehrenbach F. J. Gel-filtration behaviour and molecular weight of NAD-glycohydrolase (EC 3.2.2.5) from Streptococci in column chromatography on sephadex gels. J Chromatogr. 1969 Apr 22;41(1):43–52. doi: 10.1016/0021-9673(64)80096-0. [DOI] [PubMed] [Google Scholar]
- Fehrenbach F. J. Reinigung und Kristallisation der NAD-Glykohydrolase aus C-Streptokokken. Eur J Biochem. 1971 Jan 1;18(1):94–102. doi: 10.1111/j.1432-1033.1971.tb01219.x. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Bernheimer A. W. Purification and physical properties of group C streptococcal phage-associated lysin. J Exp Med. 1971 May 1;133(5):1105–1117. doi: 10.1084/jem.133.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN N. O., COLOWICK S. P., NASON A. Neurospora diphosphopyridine nucleotidase. J Biol Chem. 1951 Aug;191(2):473–483. [PubMed] [Google Scholar]
- LIU T. Y., NEUMANN N. P., ELLIOTT S. D., MOORE S., STEIN W. H. Chemical properties of streptococcal proteinase and its zymogen. J Biol Chem. 1963 Jan;238:251–256. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu T. Y. Demonstration of the presence of a histidine residue at the active site of streptococcal proteinase. J Biol Chem. 1967 Sep 25;242(18):4029–4032. [PubMed] [Google Scholar]
- PACE M. G., PAPPENHEIMER A. M., Jr An immunochemical study of streptococcal diphosphopyridine nucleotidase. J Immunol. 1959 Jul;83(1):83–86. [PubMed] [Google Scholar]
- SMITH E. L., STOCKELL A. Amino acid composition of crystalline carboxypeptidase. J Biol Chem. 1954 Apr;207(2):501–514. [PubMed] [Google Scholar]
- Shany S., Bernheimer A. W., Grushoff P. S., Kim K. S. Evidence for membrane cholesterol as the common binding site for cereolysin, streptolysin O and saponin. Mol Cell Biochem. 1974 May 30;3(3):179–186. doi: 10.1007/BF01686643. [DOI] [PubMed] [Google Scholar]
- Shany S., Grushoff P. S., Bernheimer A. W. Physical separation of streptococcal nicotinamide adenine dinucleotide glycohydrolase from streptolysin O. Infect Immun. 1973 May;7(5):731–734. doi: 10.1128/iai.7.5.731-734.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



