Abstract
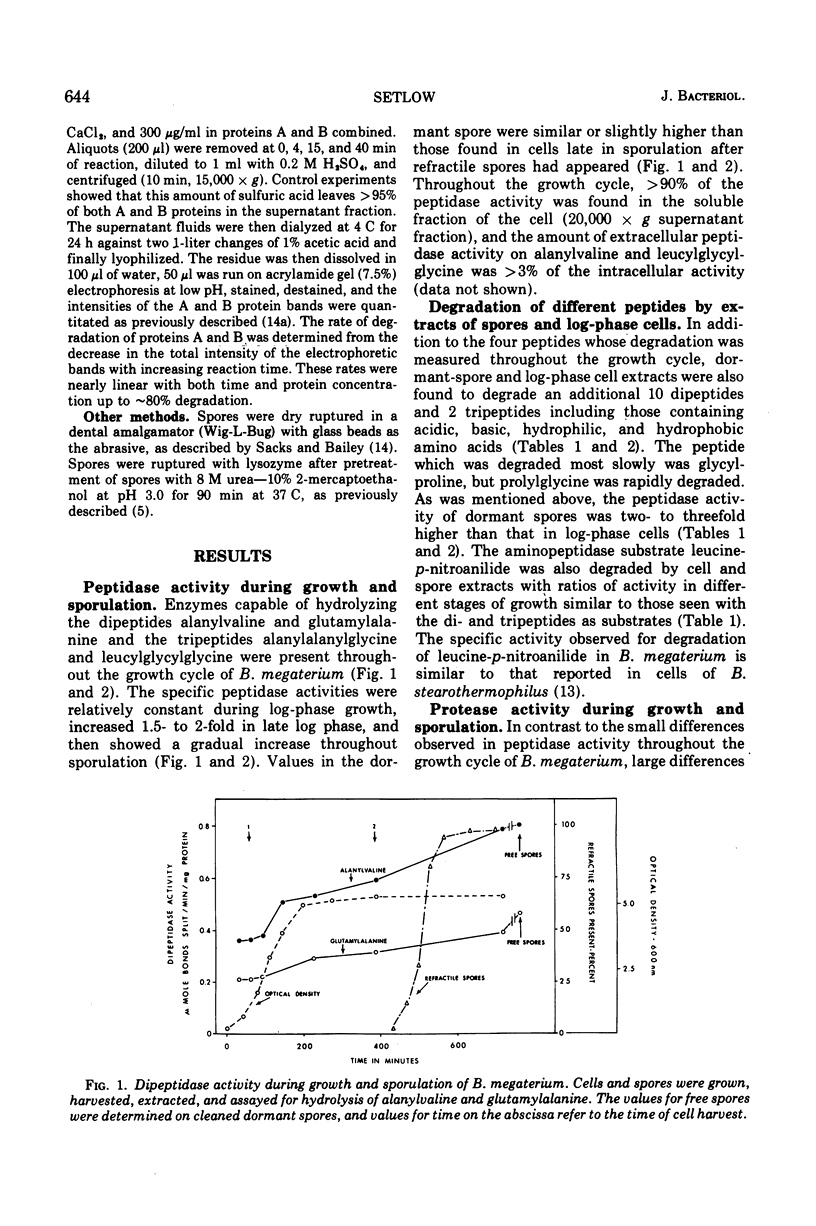
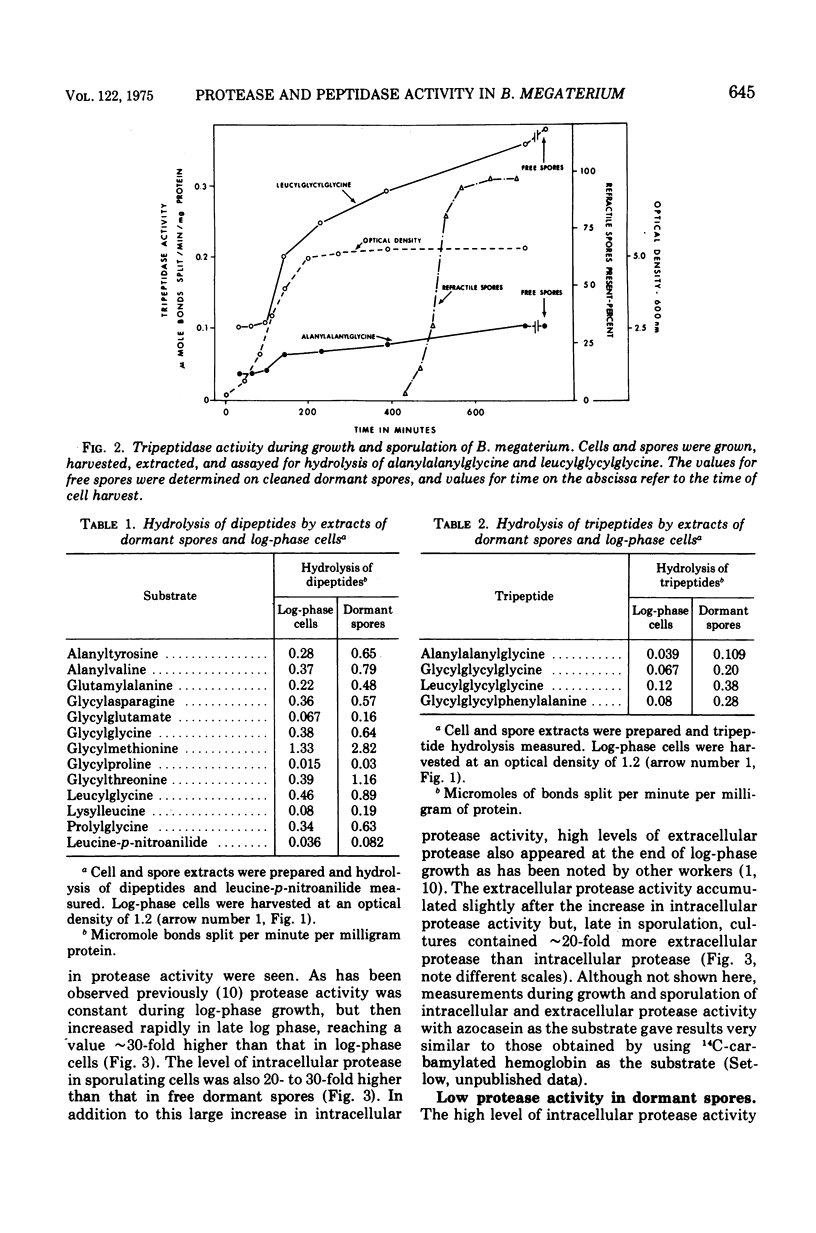
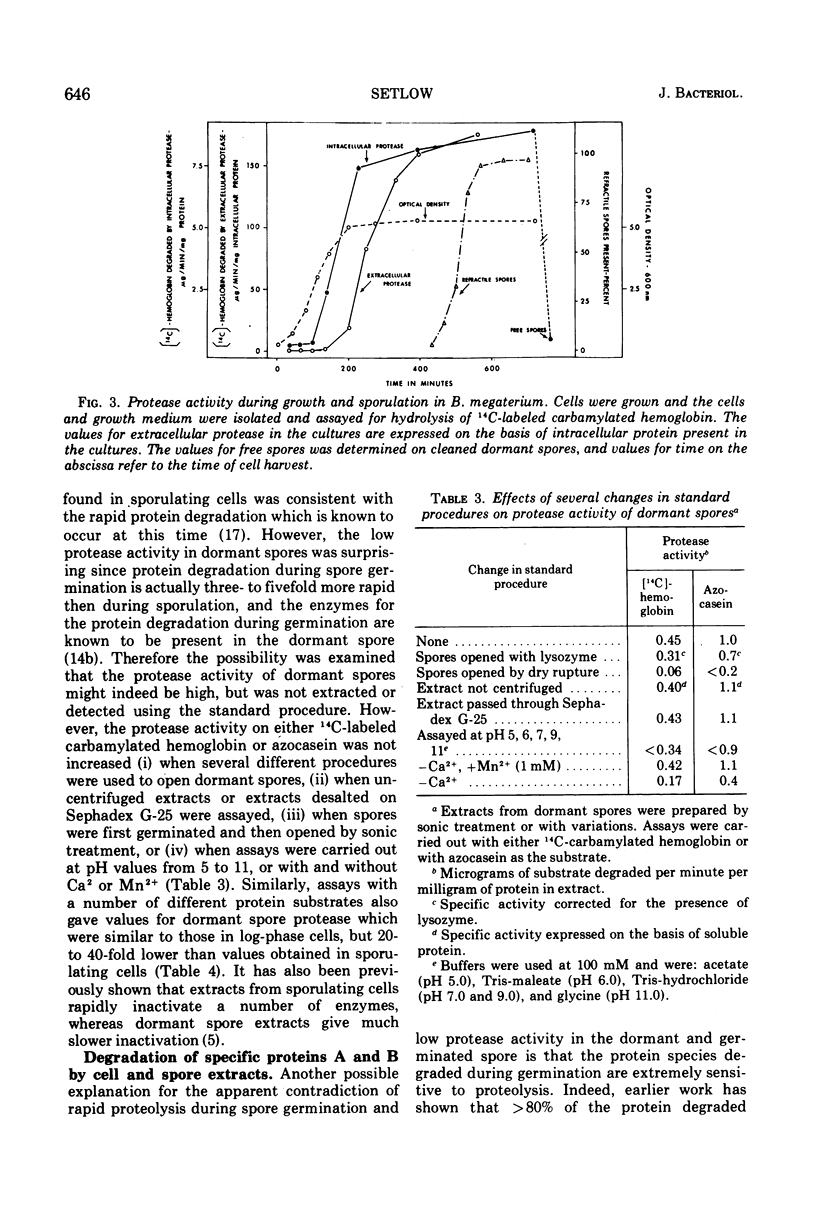
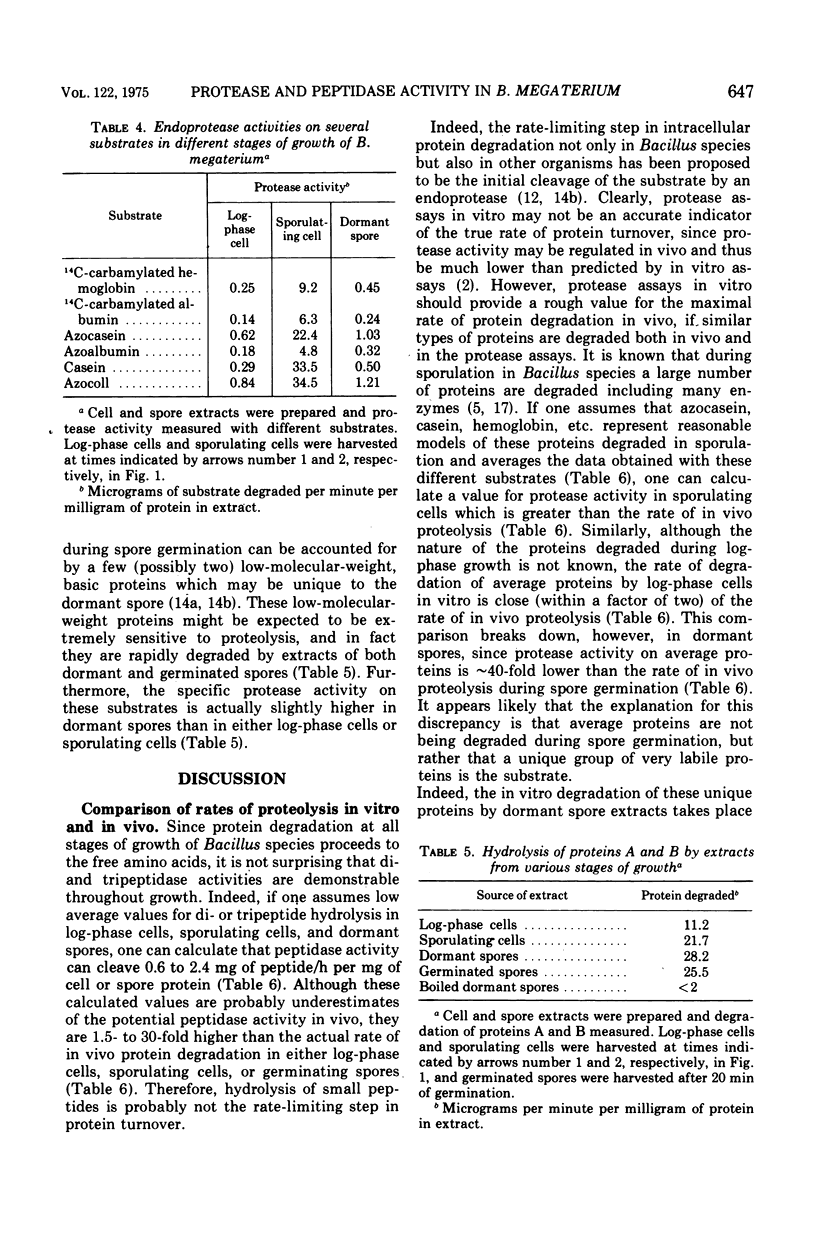
Peptidase and protease activities on many different substrates have been determined in several stages of growth of Bacillus megaterium. Extracts of log-phase cells, sporulating cells, and dormant spores of B. megaterium each hydrolyzed 16 different di- and tripeptides. The specific peptidase activity was highest in dormant spores, and the activity in sporulating cells and log-phase cells was about 1.2-fold and 2- to 3-fold lower, respectively. This peptidase acticity was wholly intracellular since extracellular peptidase activity was not detected throughout growth and sporulation. In contrast, intracellular protease activity on a variety of common protein substrates was highest in sporulating cells, and much extracellular activity was also present at this time. The specific activity of intracellular protease in sporulating cells was about 50- and 30-fold higher than that in log-phase cells and dormant spores, respectively. However, the two unique dormant spores proteins known to be the major species degraded during spore germination were degraded most rapidly by extracts of dormant spores, and slightly slower by extracts from log-phase or sporulating cells. The specific activities for degradation of peptides and proteins are compared to values for intracellular protein turnover during various stages of growth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNLOHR R. W. POSTLOGARITHMIC PHASE METABOLISM OF SPORULATING MICROORGANISMS. I. PROTEASE OF BACILLUS LICHENIFORMIS. J Biol Chem. 1964 Feb;239:538–543. [PubMed] [Google Scholar]
- Bernlohr R. W., Clark V. Characterization and regulation of protease synthesis and activity in Bacillus licheniformis. J Bacteriol. 1971 Jan;105(1):276–283. doi: 10.1128/jb.105.1.276-283.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop H. L., Doi R. H. Isolation and characterization of ribosomes from Bacillus subtilis spores. J Bacteriol. 1966 Feb;91(2):695–701. doi: 10.1128/jb.91.2.695-701.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Ulane R. Chitin synthetase activating factor from yeast, a protease. Biochem Biophys Res Commun. 1973 Jan 4;50(1):186–191. doi: 10.1016/0006-291x(73)91081-4. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. 8. Patterns of enzyme development during growth and sporulation of Baccillus subtilis. J Biol Chem. 1968 Sep 25;243(18):4653–4660. [PubMed] [Google Scholar]
- LEVINSON H. S., SEVAG M. G. Manganese and the proteolytic activity of spore extracts of Bacillus megaterium in relation to germination. J Bacteriol. 1954 May;67(5):615–616. doi: 10.1128/jb.67.5.615-616.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandelstam J., Waites W. M. Sporulation in Bacillus subtilis. The role of exoprotease. Biochem J. 1968 Oct;109(5):793–801. doi: 10.1042/bj1090793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J. Caractérisation d'une endopeptidase cytoplasmique chez Bacillus megaterium en voie de sporulation. C R Acad Sci Hebd Seances Acad Sci D. 1971 Mar 29;272(13):1806–1809. [PubMed] [Google Scholar]
- Njus D., Baldwin T. O., Hastings J. W. A sensitive assay for proteolytic enzymes using bacterial luciferase as a substrate. Anal Biochem. 1974 Sep;61(1):280–287. doi: 10.1016/0003-2697(74)90356-x. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Turnover of intracellular proteins. Annu Rev Microbiol. 1972;26:103–126. doi: 10.1146/annurev.mi.26.100172.000535. [DOI] [PubMed] [Google Scholar]
- Roncari G., Zuber H. Thermophilic aminopeptidases from Bacillus stearothermophilus. I. Isolation, specificity, and general properties of the thermostable aminopeptidase I. Int J Protein Res. 1969;1(1):45–61. doi: 10.1111/j.1399-3011.1969.tb01625.x. [DOI] [PubMed] [Google Scholar]
- SACKS L. E., BAILEY G. F. DRY RUPTURE OF BACTERIAL SPORES. J Bacteriol. 1963 Mar;85:720–721. doi: 10.1128/jb.85.3.720-721.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Identification of several unique low molecular weight basic proteins in dormant spores of Bacillus megaterium and their degradation during spore germination. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1110–1117. doi: 10.1016/s0006-291x(74)80398-0. [DOI] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XVII. Sulfhydryl and disulfide levels in dormancy and germination. J Bacteriol. 1969 Dec;100(3):1155–1160. doi: 10.1128/jb.100.3.1155-1160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Protein metabolism during germination of Bacillus megaterium spores. II. Degradation of pre-existing and newly synthesized protein. J Biol Chem. 1975 Jan 25;250(2):631–637. [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Kornberg A. Biochemical studies of bacterial sporulation and germaination. VII. Protein turnover during sporulation of Bacillus subtilis. J Biol Chem. 1968 Sep 10;243(17):4600–4605. [PubMed] [Google Scholar]


