Abstract
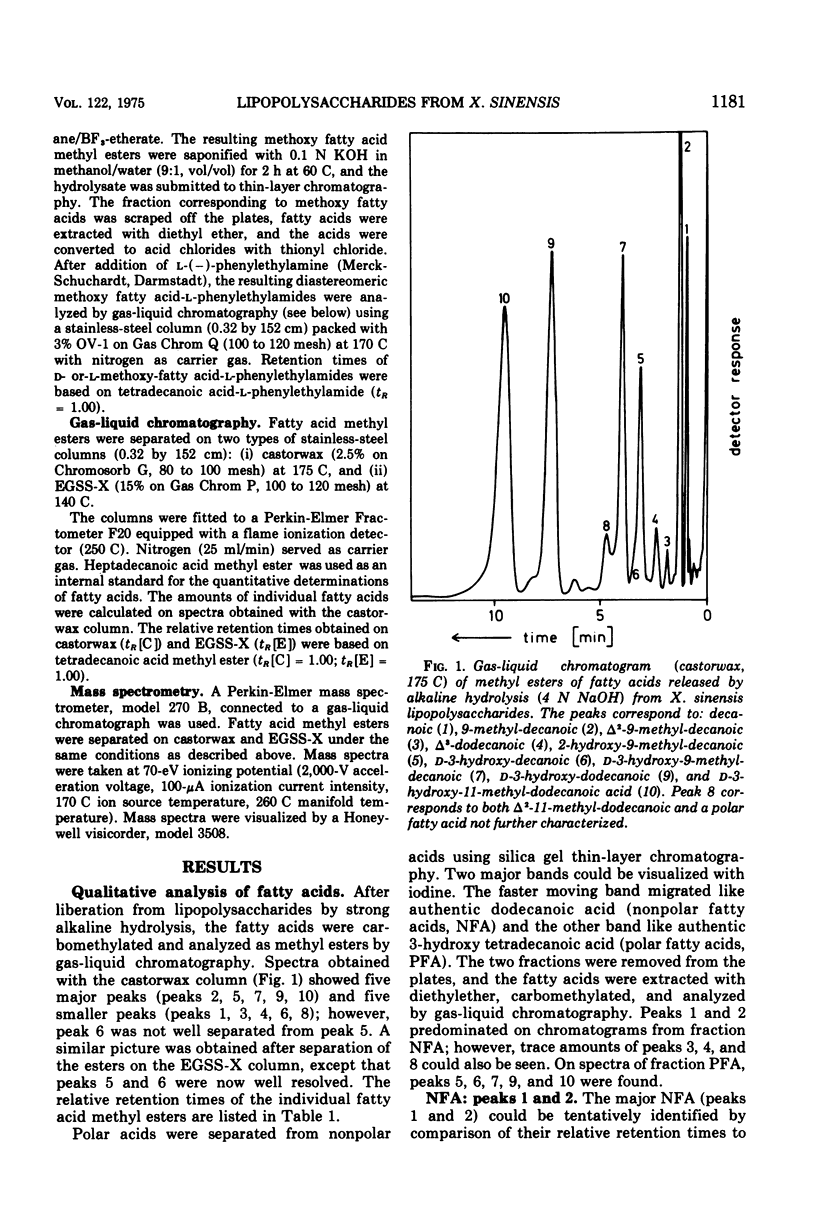
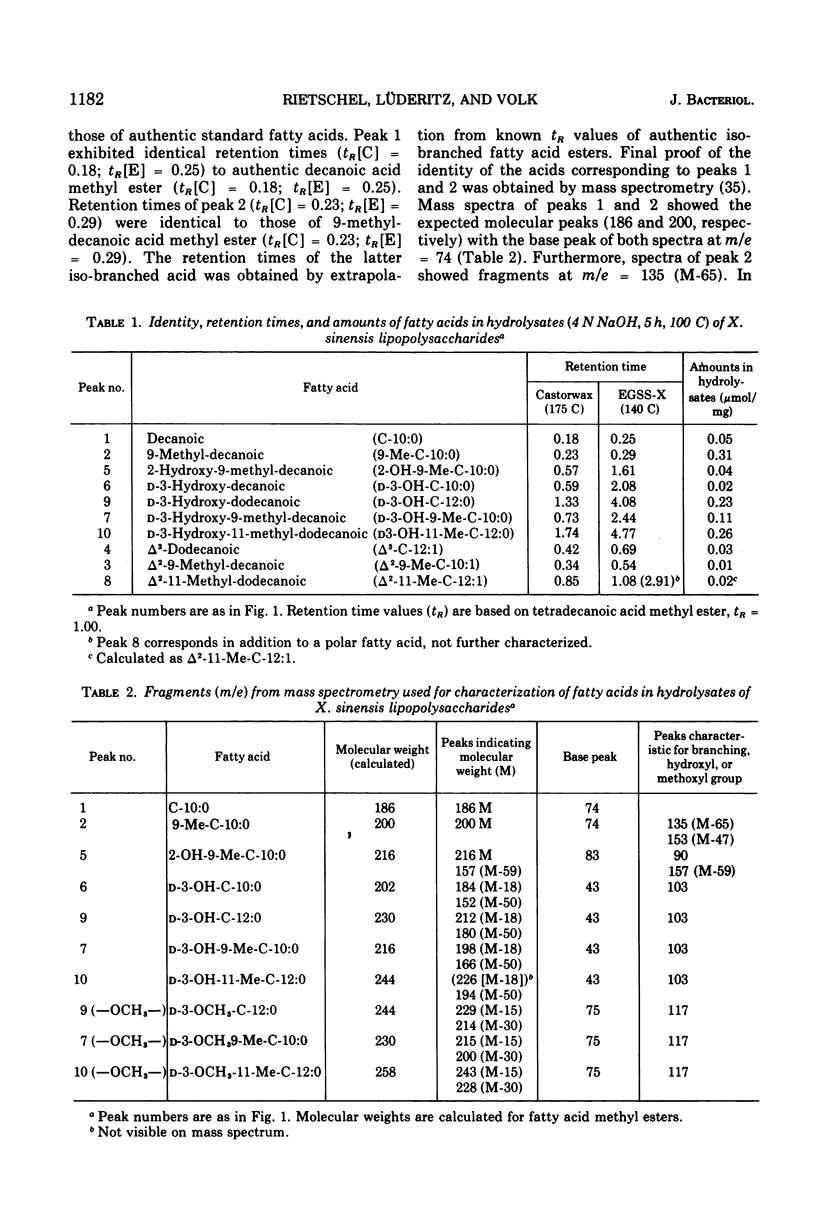
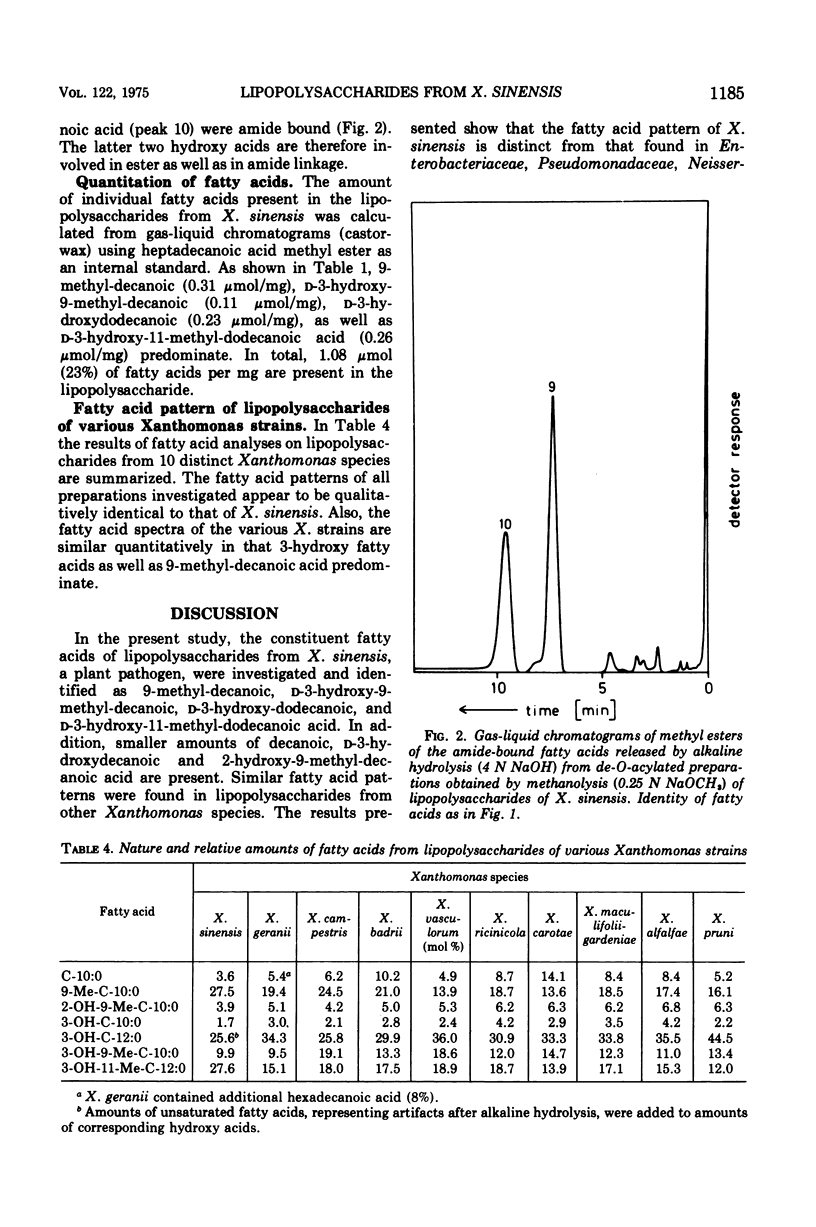
The fatty acids present in lipopolysaccharides from Xanthomonas sinensis were identified as decanoic, 9-methyl-decanoic, 2-hydroxy-9-methyl-decanoic, 2-hydroxy-9-methyl-decanoic, D-3-hydroxy-decanoic, D-3-hydroxy-9-methyl-decanoic, D-3-hydroxy-dodecanoic, and D-3-hydroxy-11-methyl-dodecanoic acid. These fatty acids occur in the lipid A component where they are bound through ester and amide linkages to glucosamine residues. All types of fatty acids are ester bound; however, part of D-3-hydroxy-dodecanoic and D-3-hydroxy-11-methyl-dodecanoic acid is also involved in amide linkage. The hydroxyl groups of ester-linked 3-hydroxy fatty acids are not substituted. Similar fatty acid patterns were obtained from lipopolysaccharides of nine other Xanthomonas species.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. A., Kates M., Shaw D. H., Yaguchi M. Studies on the chemical constitution of cell-wall lipopolysaccharides from Neisseria perflava. Can J Biochem. 1968 Oct;46(10):1175–1184. doi: 10.1139/o68-176. [DOI] [PubMed] [Google Scholar]
- Adams G. A., Quadling C., Yaguchi M., Tornabene T. G. The chemical composition of cell-wall lipopolysaccharides from Moraxella duplex and Micrococcus calco-aceticus. Can J Microbiol. 1970 Jan;16(1):1–8. doi: 10.1139/m70-001. [DOI] [PubMed] [Google Scholar]
- Adams G. A., Singh P. P. The chemical constitution of lipid A from Serratia marcescens. Can J Biochem. 1970 Jan;48(1):55–62. doi: 10.1139/o70-010. [DOI] [PubMed] [Google Scholar]
- Armstrong I. L., Redmond J. W. The fatty acids present in the lipopolysaccharide of Vibrio cholerae 569B (Inaba). Biochim Biophys Acta. 1974 May 29;348(2):302–305. doi: 10.1016/0005-2760(74)90242-2. [DOI] [PubMed] [Google Scholar]
- Baca O. G., Paretsky D. Partial chemical characterization of a toxic lipopolysaccharide from Coxiella burneti. Infect Immun. 1974 May;9(5):959–961. doi: 10.1128/iai.9.5.959-961.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F. M., Fukui G. M., Ludwig B. J., Rosselet J. P. Increased host resistance to infection elicited by lipopolysaccharides from Brucella abortus. Proc Soc Exp Biol Med. 1969 Sep;131(4):1376–1381. doi: 10.3181/00379727-131-34111. [DOI] [PubMed] [Google Scholar]
- Bishop D. G., Hewett M. J., Knox K. W. Biochemical studies on lipopolysaccharides of Veillonella. Eur J Biochem. 1971 Mar 11;19(2):169–175. doi: 10.1111/j.1432-1033.1971.tb01301.x. [DOI] [PubMed] [Google Scholar]
- Bishop D. G., Hewett M. J., Knox K. W. Occurrence of 3-hydroxytridecanoic and 3-hydroxypentadecanoic acids in the lipopolysaccharides of Veillonella. Biochim Biophys Acta. 1971 Mar 16;231(2):274–276. doi: 10.1016/0005-2760(71)90138-x. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Fensom A. H., Gray G. W. The chemical composition of the lipopolyacarideof Pseudomonas aeruginosa. Biochem J. 1969 Sep;114(2):185–196. doi: 10.1042/bj1140185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O. Interaction of lipopolysaccharides and lipid A with complement. Eur J Biochem. 1971 Mar 1;19(1):143–152. doi: 10.1111/j.1432-1033.1971.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O., Kim Y. B., Watson D. W. Biological activities of lipid A complexed with bovine-serum albumin. Eur J Biochem. 1972 Dec 4;31(2):230–233. doi: 10.1111/j.1432-1033.1972.tb02524.x. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., O'Leary W. M. Lipoidal components of bacterial lipopolysaccharides: nature and distribution of fatty acids in Aerobacter aerogenes. J Bacteriol. 1968 Sep;96(3):660–664. doi: 10.1128/jb.96.3.660-664.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock I. C., Humphreys G. O., Meadow P. M. Characterisation of the hydroxy acids of Pseudomonas aeruginosa 8602. Biochim Biophys Acta. 1970 Mar 10;202(2):389–391. doi: 10.1016/0005-2760(70)90204-3. [DOI] [PubMed] [Google Scholar]
- Jann B., Jann K., Beyaert G. O. 2-Amino-2,6-dideoxy-d-glucose (D-quinovosamine): a constituent of the lipopolysaccharides of Vibrio cholerae. Eur J Biochem. 1973 Sep 3;37(3):531–534. doi: 10.1111/j.1432-1033.1973.tb03015.x. [DOI] [PubMed] [Google Scholar]
- KANESHIRO T., MARR A. G. Hydroxy fatty acids of Azotobacter agilis. Biochim Biophys Acta. 1963 Jun 18;70:271–277. doi: 10.1016/0006-3002(63)90751-0. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A., Pascher I. Resolution and chromatographic configuration analysis of 2-hydroxy fatty acids. Chem Phys Lipids. 1974 Apr;12(2):65–74. doi: 10.1016/0009-3084(74)90046-2. [DOI] [PubMed] [Google Scholar]
- Key B. A., Gray G. W., Wilkinson S. G. The purification and chemical composition of the lipopolysaccharide of Pseudomonas alcaligenes. Biochem J. 1970 Dec;120(3):559–566. doi: 10.1042/bj1200559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeltzow D. E., Conrad H. E. Structural heterogeneity in the lipopolysaccharide of Aerobacter aerogenes NCTC 243. Biochemistry. 1971 Jan 19;10(2):214–224. doi: 10.1021/bi00778a004. [DOI] [PubMed] [Google Scholar]
- Lacave C., Asselineau J., Serre A., Roux J. Comparaison de la composition chimique d'une fraction lipopolysaccharidique et d'une fraction polysaccharidique isolées de Brucella melitensis. Eur J Biochem. 1969 Jun;9(2):189–198. doi: 10.1111/j.1432-1033.1969.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Lugowski C., Romanowska E. Chemical studies on Shigella sonnei lipid A. Eur J Biochem. 1974 Oct 2;48(2):319–323. doi: 10.1111/j.1432-1033.1974.tb03771.x. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Samuels S. B., Liddle J., McKinney R. M. Occurrence of branched-cahin hydroxy fatty acids in Pseudomonas maltophilia. J Bacteriol. 1973 Jun;114(3):1018–1024. doi: 10.1128/jb.114.3.1018-1024.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESBITT J. A., 3rd, LENNARZ W. J. COMPARISON OF LIPIDS AND LIPOPOLYSACCHARIDE FROM THE BACILLARY AND L FORMS OF PROTEUS P18. J Bacteriol. 1965 Apr;89:1020–1025. doi: 10.1128/jb.89.4.1020-1025.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYHAGE R., STENHAGEN E. Mass spectrometry in lipid research. J Lipid Res. 1960 Oct;1:361–390. [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Kim Y. B., Watson D. W., Galanos C., Lüderitz O., Westphal O. Pyrogenicity and immunogenicity of lipid A complexed with bovine serum albumin or human serum albumin. Infect Immun. 1973 Aug;8(2):173–177. doi: 10.1128/iai.8.2.173-177.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Palin W. J., Watson D. W. Nature and linkages of the fatty acids present in lipopolysaccharides from Vibrio metchnikovii and Vibrio parahemolyticus. Eur J Biochem. 1973 Aug 1;37(1):116–120. doi: 10.1111/j.1432-1033.1973.tb02965.x. [DOI] [PubMed] [Google Scholar]
- Rooney S. A., Goldfine H., Sweeley C. C. The identification of trans-2-tetradecenoic acid in hydrolysates of lipid A from Escherichia coli. Biochim Biophys Acta. 1972 Jul 7;270(3):289–295. doi: 10.1016/0005-2760(72)90192-0. [DOI] [PubMed] [Google Scholar]
- Rosenfelder G., Lüderitz O., Westphal O. Composition of lipopolysaccharides from Myxococcus fulvus and other fruiting and non-fruiting myxobacteria. Eur J Biochem. 1974 May 15;44(2):411–420. doi: 10.1111/j.1432-1033.1974.tb03499.x. [DOI] [PubMed] [Google Scholar]
- Steiner S., Conti S. F., Lester R. L. Occurrence of phosphonosphingolipids in Bdellovibrio bacteriovorus strain UKi2. J Bacteriol. 1973 Dec;116(3):1199–1211. doi: 10.1128/jb.116.3.1199-1211.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Thornley M. J., Glauert A. M. Chemical analysis of the outer membrane and other layers of the cell envelope of Acinetobacter sp. J Bacteriol. 1973 Oct;116(1):410–417. doi: 10.1128/jb.116.1.410-417.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk W. A. Cell wall lipopolysaccharides from xanthomonas species. J Bacteriol. 1966 Jan;91(1):39–42. doi: 10.1128/jb.91.1.39-42.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk W. A. Isolation of D-galacturonic acid 1-phosphate from hydrolysates of cell wall lipopolysaccharide extracted from Xanthomonas campestris. J Bacteriol. 1968 Mar;95(3):782–786. doi: 10.1128/jb.95.3.782-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk W. A. Quantitative assay of polysaccharide components obtained from cell wall lipopolysaccharides of Xanthomonas species. J Bacteriol. 1968 Mar;95(3):980–982. doi: 10.1128/jb.95.3.980-982.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON D. W., KIM Y. B. MODIFICATION OF HOST RESPONSES TO BACTERIAL ENDOTOXINS. I. SPECIFICITY OF PYROGENIC TOLERANCE AND THE ROLE OF HYPERSENSITIVITY IN PYROGENICITY, LETHALITY, AND SKIN REACTIVITY. J Exp Med. 1963 Sep 1;118:425–446. doi: 10.1084/jem.118.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckesser J., Drews G., Fromme I. Chemical analysis of and degradation studies on the cell wall lipopolysaccharide of Rhodopseudomonas capsulata. J Bacteriol. 1972 Mar;109(3):1106–1113. doi: 10.1128/jb.109.3.1106-1113.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S. G., Galbraith L., Lightfoot G. A. Cell walls, lipids, and lipopolysaccharides of Pseudomonas species. Eur J Biochem. 1973 Feb 15;33(1):158–174. doi: 10.1111/j.1432-1033.1973.tb02666.x. [DOI] [PubMed] [Google Scholar]


