Abstract
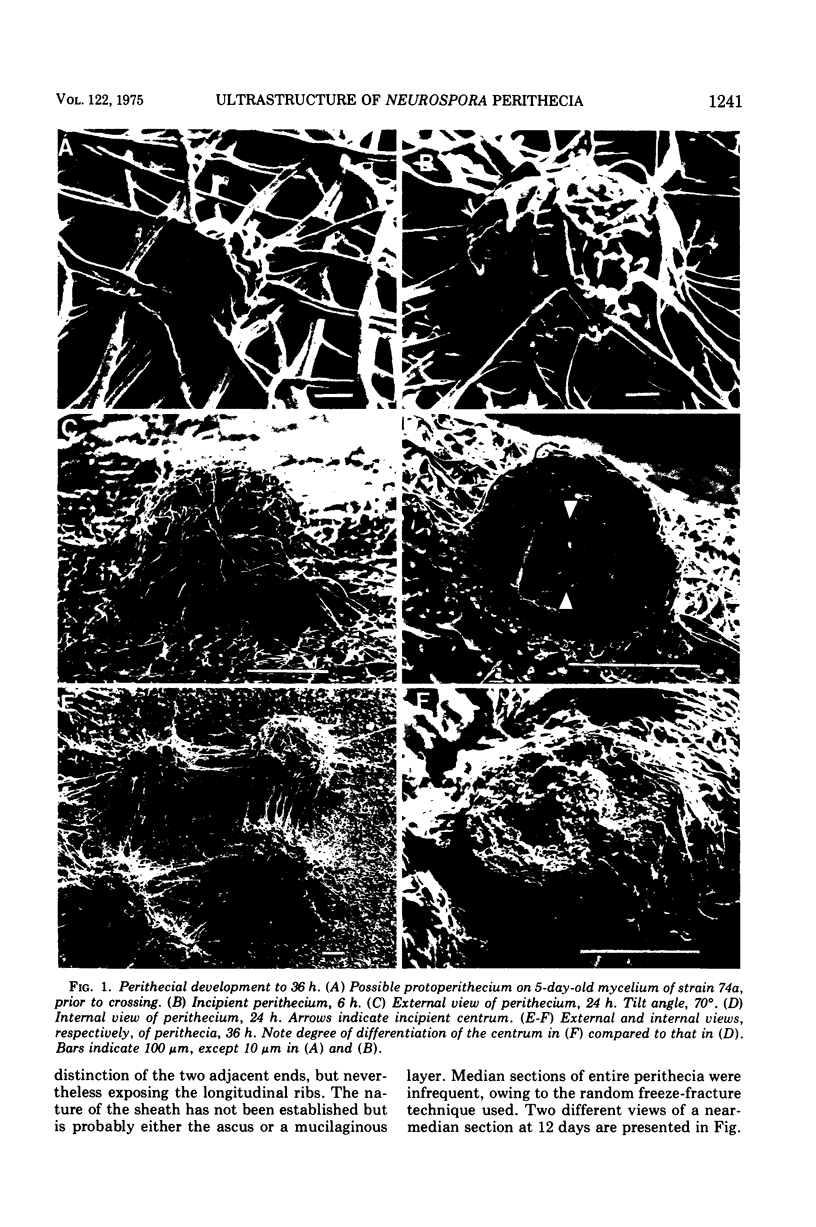
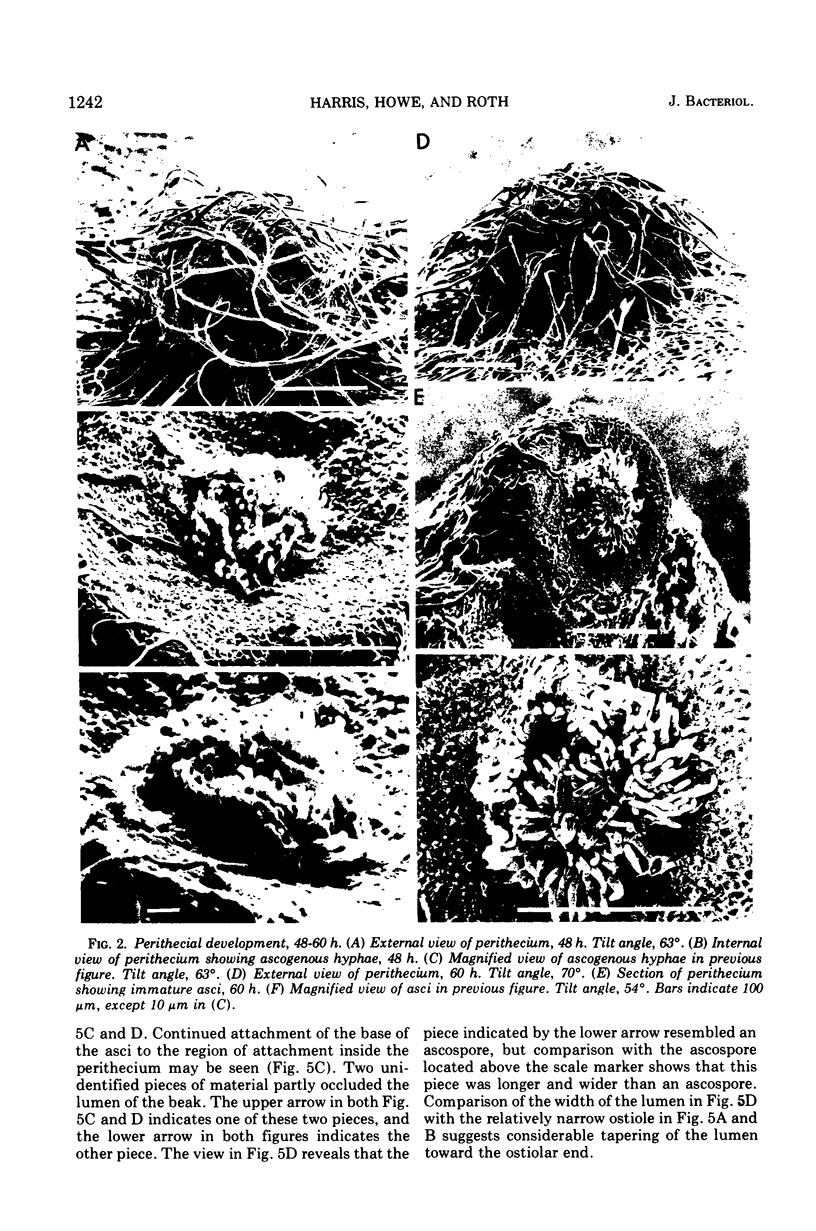
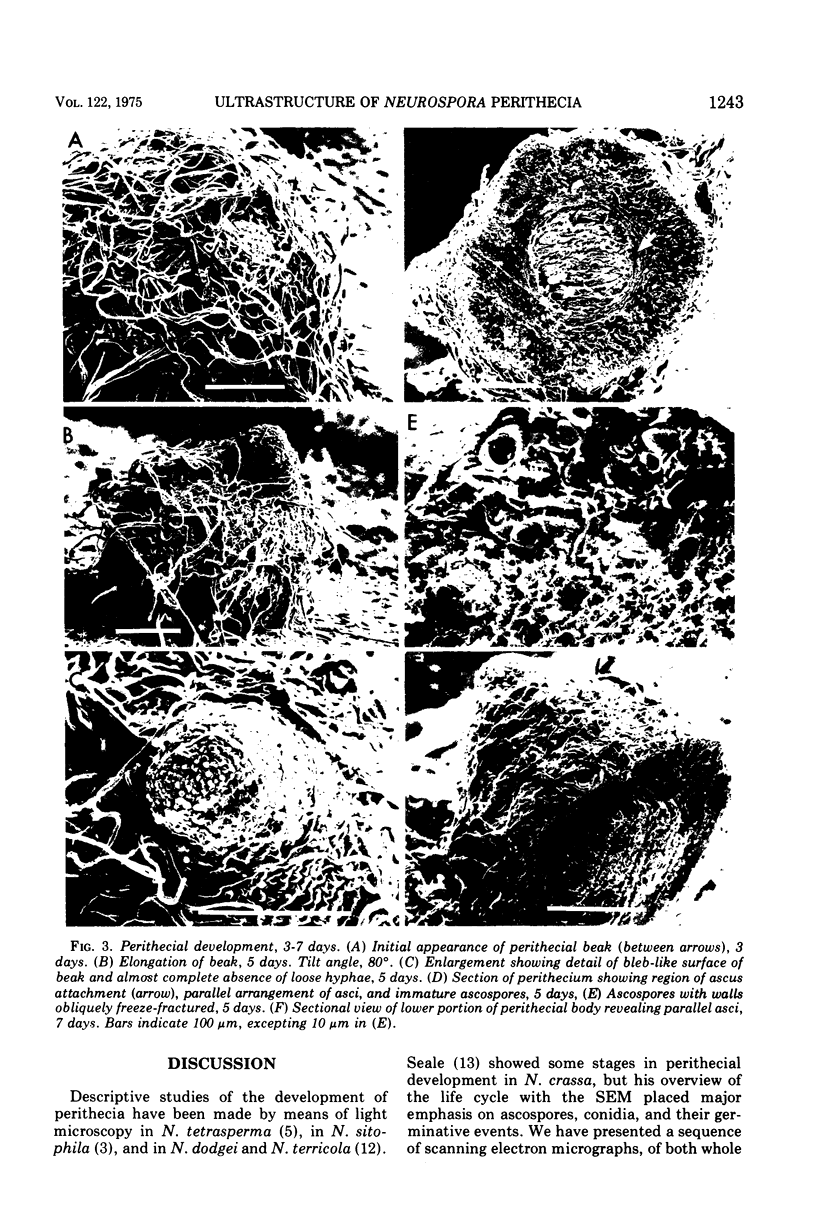
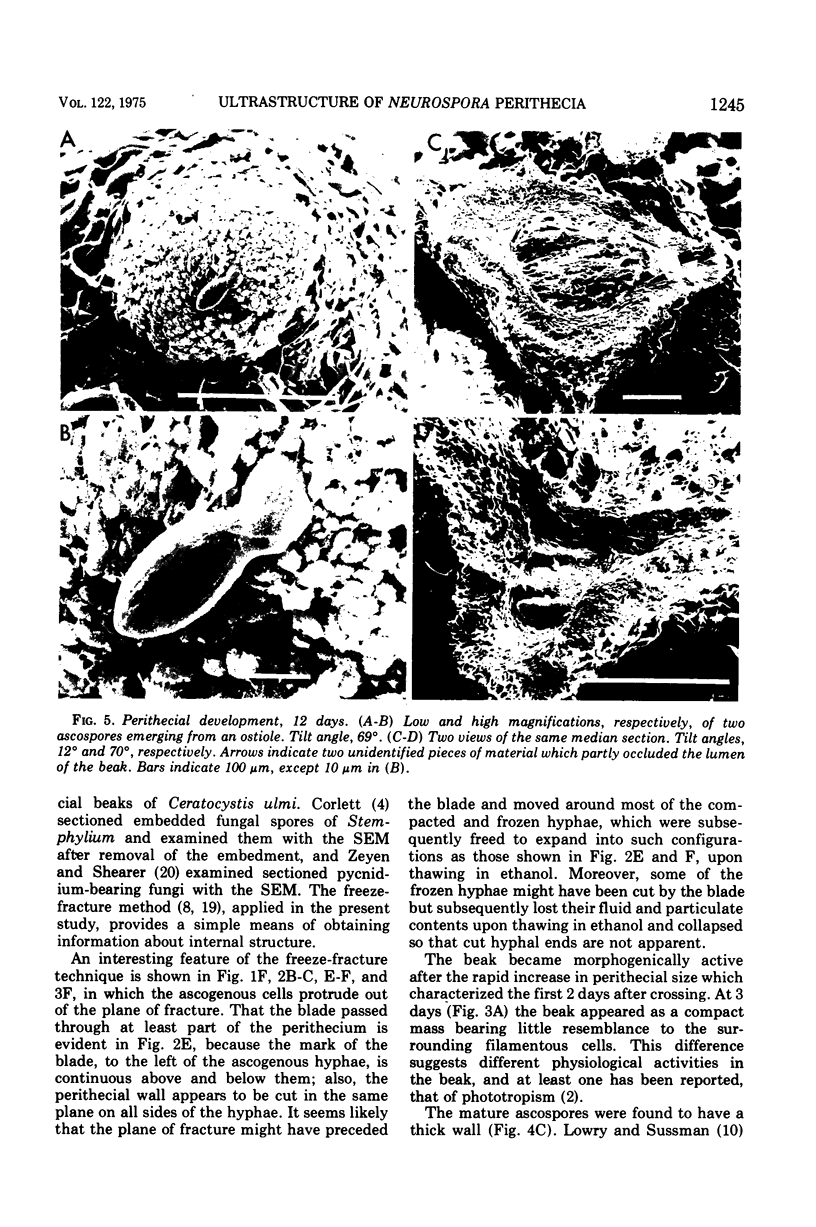
Stages in the development of perithecia of Neurospora crassa, designated by the time elapsed after crossing, were investigated with the scanning electron microscope, from protoperithecia through perithecia. The usual examination of external features of whole specimens with this instrument was augmented by a freeze-fracture technique which allowed the viewing of development internally as well. Rapid increases in perithecial size soon after crossing were followed by the appearance, in section, of a centrum, at first undifferentiated but subsequently developing ascogenous hyphae. The perithecial beak appeared as a compact mass easily distinguishable in whole specimens from the surrounding hyphae by means of texture as well as shape. Two ascospores were photographed during emergence from an ostiole, but ostioles were found more frequently closed than open.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin W. L., Frederick L., Roth I. L. Scanning electron microscope studies on ascospores of homothallic species of Neurospora. Mycologia. 1974 Jan-Feb;66(1):130–138. [PubMed] [Google Scholar]
- Corlett M. Surface structure of the conidium and conidiophore of Stemphylium botryosum. Can J Microbiol. 1973 Mar;19(3):392–393. doi: 10.1139/m73-064. [DOI] [PubMed] [Google Scholar]
- Howe H. B., Jr, Benson E. W. A perithecial color mutant of Neurospora crassa. Mol Gen Genet. 1974;131(1):79–83. doi: 10.1007/BF00269389. [DOI] [PubMed] [Google Scholar]
- Lowry R. J., Sussman A. S. Ultrastructural changes during germination of ascospores of Neurospora tetrasperma. J Gen Microbiol. 1968 May;51(3):403–409. doi: 10.1099/00221287-51-3-403. [DOI] [PubMed] [Google Scholar]
- Nasrallah J. B., Srb A. M. Genetically related protein variants specifically associated with fruiting body maturation in Neurospora. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1891–1893. doi: 10.1073/pnas.70.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A. C., Backus M. P. Ascocarp development in two homothallic neurosporas. Mycologia. 1968 Jan-Feb;60(1):16–28. [PubMed] [Google Scholar]
- Seale T. Life cycle of Neurospora crassa viewed by scanning electron microscopy. J Bacteriol. 1973 Feb;113(2):1015–1025. doi: 10.1128/jb.113.2.1015-1025.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. L., Wagner P. C., DeBusk A. G. Some observations of ascospores of Neurospora crassa made with a scanning electron microscope. J Bacteriol. 1972 Sep;111(3):825–826. doi: 10.1128/jb.111.3.825-826.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis G., Wolfinbarger L., Jr, Stuart W. D., DeBusk A. G. Disruption of an amino acid transport mutant of Neurospora crassa by KCl. J Bacteriol. 1972 Feb;109(2):933–935. doi: 10.1128/jb.109.2.933-935.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanath-Reddy M., Turian G. Temperature-induced synchronous differentiation of ascogonia in Neurospora. Experientia. 1972 Jan 15;28(1):99–100. doi: 10.1007/BF01928288. [DOI] [PubMed] [Google Scholar]
- Wodzicki T. J., Humphreys W. J. Cytodifferentiation of maturing pine tracheids: the final stage. Tissue Cell. 1972;4(3):525–528. doi: 10.1016/s0040-8166(72)80028-4. [DOI] [PubMed] [Google Scholar]