Abstract
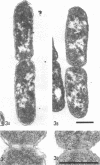
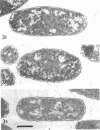
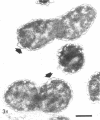
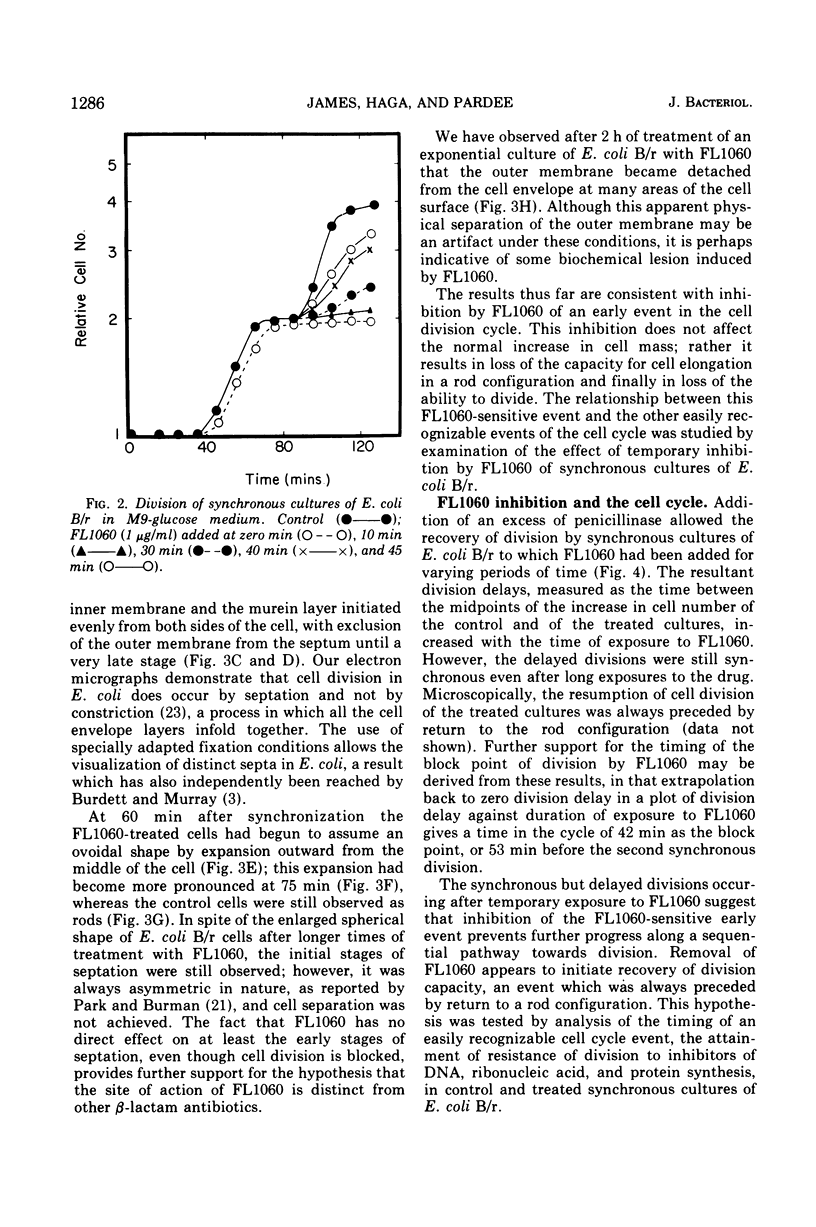
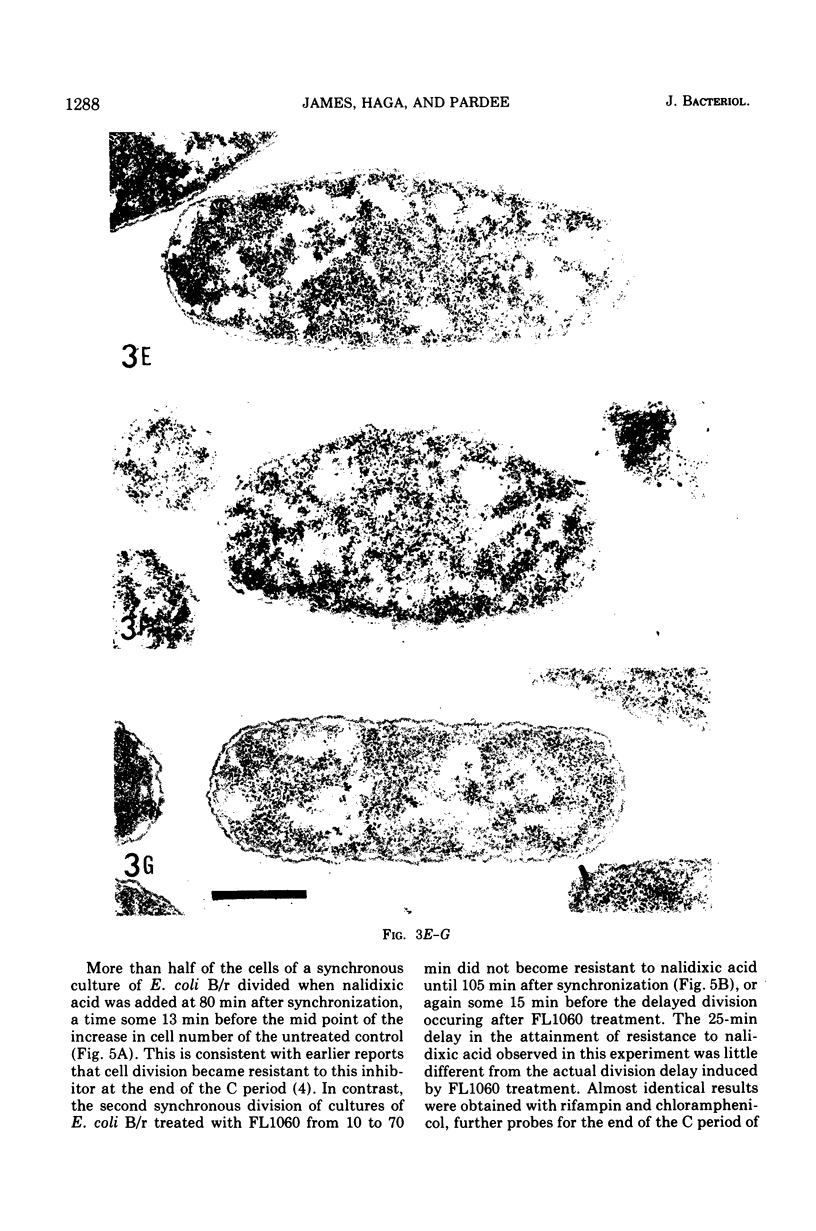
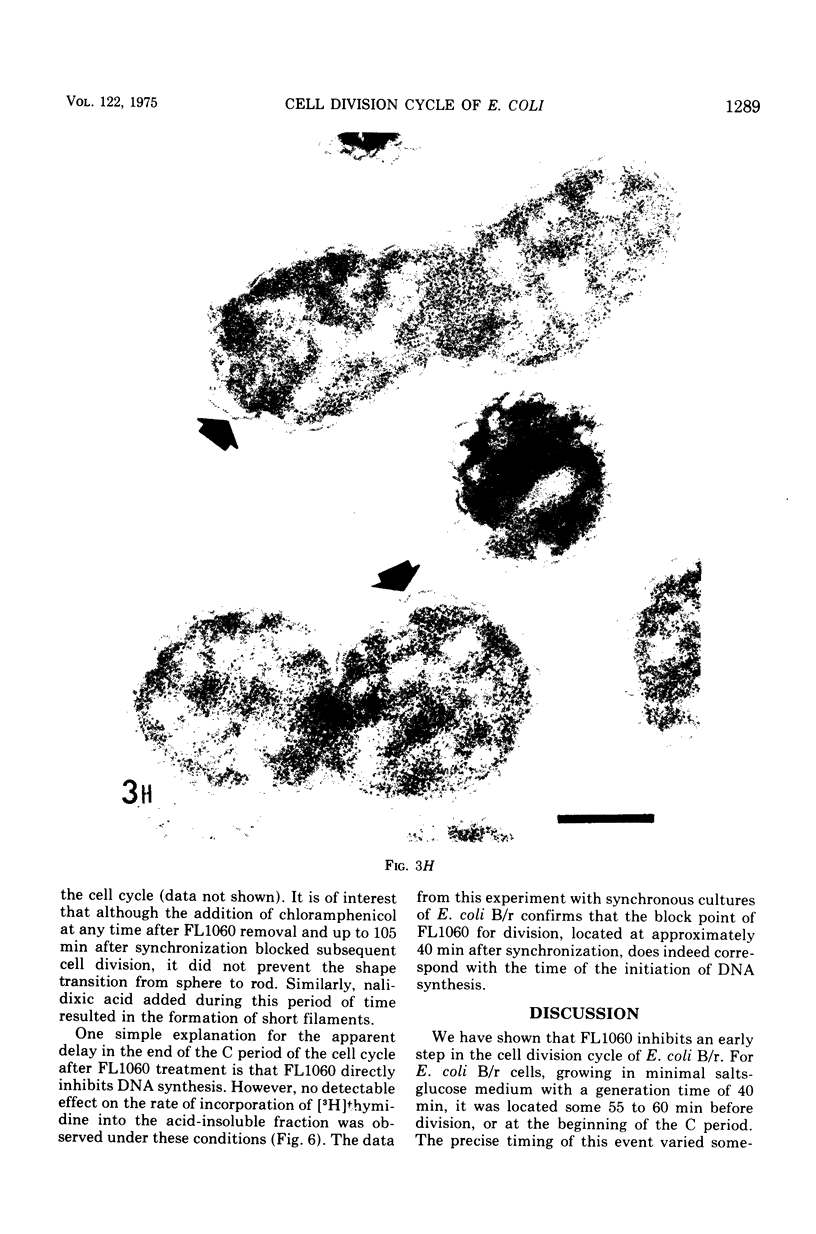
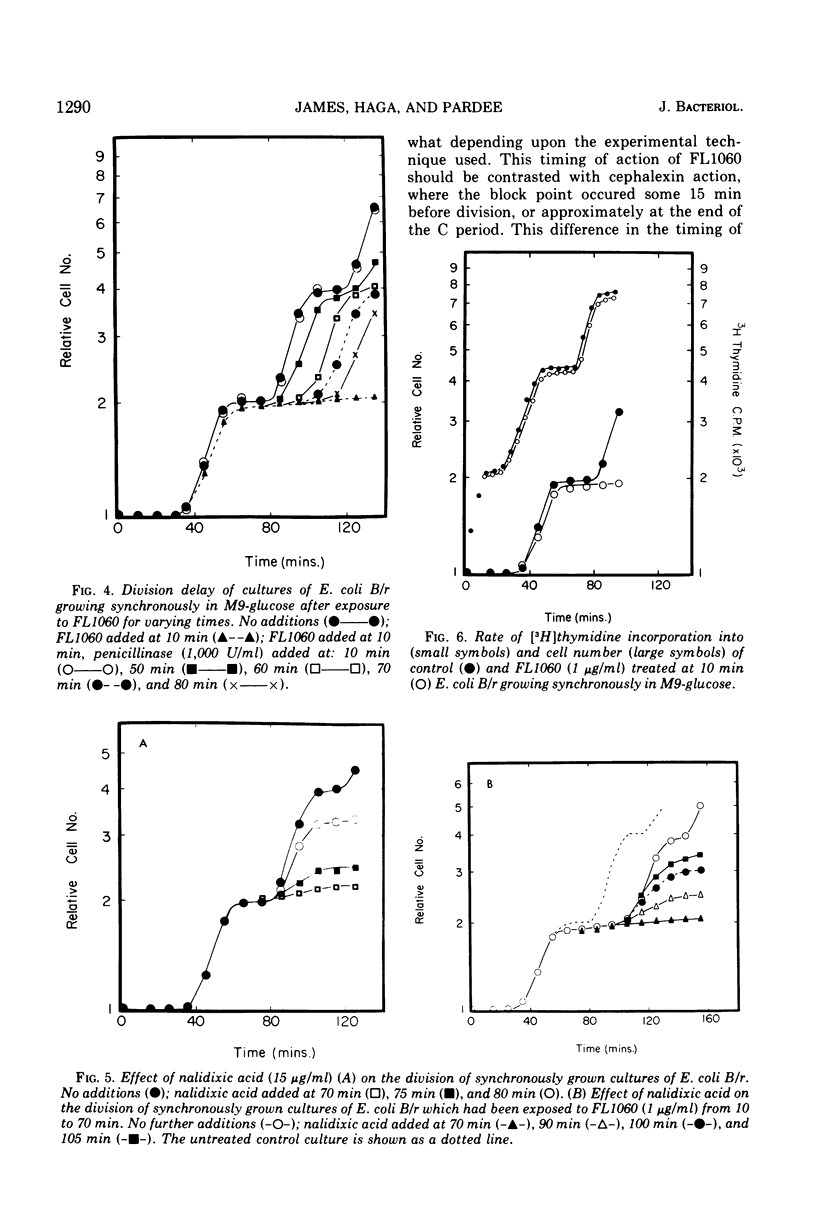
Analysis of exponential and synchronous cultures of Escherichia coli B/r after the addition of FL1060 indicates a block point for division by this agent some 15 to 20 min before the end of the preceding cell division cycle, a time corresponding to the beginning of the C period of the cell division cycle. Morphological examination of FL1060-treated synchronous cultures of E. coli /r was consistent with inhibition by FL1060 of a very early event in the cell division cycle. This event appears to be essential for normal cell surface elongation in a rod configuration. Temporary treatment of synchronous cultures of E. coli B/r with FL1060 resulted in division delay, the extent of which was a function of the duration of exposure to FL1060. However, even after relatively long times of FL1060 treatment the delayed divisions were still synchronous. Although FL1060 had no direct effect on deoxyribonucleic acid (DNA) synthesis, the synchronous delayed division occuring after temporary treatment with FL1060 were accompanied by a delay in the attainment of resistance of cell division to inhibitors of DNA, ribonucleic acid, and protein synthesis. These results suggest aht an FL1060-sensitive event initiates at the beginning of the C period of the cell division cycle of E. coli and is responsible for normal cell elongation. This cell elongation pathway procedes independently of DNA synthesis, but there is an interaction between this pathway and termination of a round of DNA replication in which a normal rod configuration is necessary to allow a signal for cell division to be generated upon completion of DNA replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V., Wolff H. The murein-lipoprotein linkage in the cell wall of Escherichia coli. Eur J Biochem. 1970 Jun;14(2):387–391. doi: 10.1111/j.1432-1033.1970.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Bricas E., Dezélée P. Sur l'idenité de la mucoendopeptidase et de la carboxy-peptidase I d'Escherichia coli, eneymes hydrolysant des liaisons de configuration D-D et inhibées par la pénicilline. C R Acad Sci Hebd Seances Acad Sci D. 1969;269(3):390–393. [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Septum formation in Escherichia coli: characterization of septal structure and the effects of antibiotics on cell division. J Bacteriol. 1974 Jul;119(1):303–324. doi: 10.1128/jb.119.1.303-324.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix D. E., Helmstetter C. E. Coupling between chromosome completion and cell division in Escherichia coli. J Bacteriol. 1973 Sep;115(3):786–795. doi: 10.1128/jb.115.3.786-795.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D., O'Grady F. FL 1060: a new beta-lactam antibiotic with novel properties. J Clin Pathol. 1973 Jan;26(1):1–6. doi: 10.1136/jcp.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. Deoxyribonucleic acid synthesis during the division cycle of Escherichia coli: a comparison of strains B-r, K-12, 15, and 15T- under conditions of slow growth. J Bacteriol. 1974 Mar;117(3):1216–1223. doi: 10.1128/jb.117.3.1216-1223.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter C. E. DNA synthesis during the division cycle of rapidly growing Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):507–518. doi: 10.1016/0022-2836(68)90424-5. [DOI] [PubMed] [Google Scholar]
- Hirashima A., Wu H. C., Venkateswaran P. S., Inouye M. Two forms of a structural lipoprotein in the envelope of Escherichia coli. Further characterization of the free form. J Biol Chem. 1973 Aug 25;248(16):5654–5659. [PubMed] [Google Scholar]
- Hoffmann B., Messer W., Schwarz U. Regulation of polar cap formation in the life cycle of Escherichia coli. J Supramol Struct. 1972;1(1):29–37. doi: 10.1002/jss.400010105. [DOI] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Izaki K., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XIV. Purification and properties of two D-alanine carboxypeptidases from Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3193–3201. [PubMed] [Google Scholar]
- Jones N. C., Donachie W. D. Chromosome replication, transcription and control of cell division in Escherichia coli. Nat New Biol. 1973 May 23;243(125):100–103. [PubMed] [Google Scholar]
- Jones N. C., Donachie W. D. Protein synthesis and the release of the replicated chromosome from the cell membrane. Nature. 1974 Sep 20;251(5472):252–254. doi: 10.1038/251252a0. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Renger H. Initiation of DNA replication in Escherichia coli 15T-: chronological dissection of three physiological processes required for initiation. J Mol Biol. 1969 Jun 14;42(2):221–235. doi: 10.1016/0022-2836(69)90039-4. [DOI] [PubMed] [Google Scholar]
- Lund F., Tybring L. 6 -amidinopenicillanic acids--a new group of antibiotics. Nat New Biol. 1972 Apr 5;236(66):135–137. doi: 10.1038/newbio236135a0. [DOI] [PubMed] [Google Scholar]
- Matsuhashi S., Kamiryo T., Blumberg P. M., Linnett P., Willoughby E., Strominger J. L. Mechanism of action and development of resistance to a new amidino penicillin. J Bacteriol. 1974 Feb;117(2):578–587. doi: 10.1128/jb.117.2.578-587.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior N. H., Blom J., Tybring L., Birch-Andersen A. Light and electron microscopy of the early response of Escherichia coli to a 6beta-amidinopenicillanic acid (FL 1060). Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Aug;81(4):393–407. doi: 10.1111/j.1699-0463.1973.tb02222.x. [DOI] [PubMed] [Google Scholar]
- Nishino T., Nakazawa S. Cephalexin-induced morphological alterations in the surface structures of Staphylococcus aureus and Escherichia coli demonstrated by scanning electron microscopy. Jpn J Microbiol. 1973 Sep;17(5):383–392. doi: 10.1111/j.1348-0421.1973.tb00789.x. [DOI] [PubMed] [Google Scholar]
- Park J. T., Burman L. FL-1060: a new penicillin with a unique mode of action. Biochem Biophys Res Commun. 1973 Apr 16;51(4):863–868. doi: 10.1016/0006-291x(73)90006-5. [DOI] [PubMed] [Google Scholar]
- Pierucci O., Helmstetter C. E. Chromosome replication, protein synthesis and cell division in Escherichia coli. Fed Proc. 1969 Nov-Dec;28(6):1755–1760. [PubMed] [Google Scholar]
- Steed P., Murray R. G. The cell wall and cell division of gram-negative bacteria. Can J Microbiol. 1966 Apr;12(2):263–270. doi: 10.1139/m66-036. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Willoughby E., Kamiryo T., Blumberg P. M., Yocum R. R. Penicillin-sensitive enzymes and penicillin-binding components in bacterial cells. Ann N Y Acad Sci. 1974 May 10;235(0):210–224. doi: 10.1111/j.1749-6632.1974.tb43267.x. [DOI] [PubMed] [Google Scholar]
- Suginaka H., Blumberg P. M., Strominger J. L. Multiple penicillin-binding components in Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, and Escherichia coli. J Biol Chem. 1972 Sep 10;247(17):5279–5288. [PubMed] [Google Scholar]
- Ward C. B., Glaser D. A. Correlation between rate of cell growth and rate of DNA synthesis in Escherichia coli B-r. Proc Natl Acad Sci U S A. 1971 May;68(5):1061–1064. doi: 10.1073/pnas.68.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]