Abstract
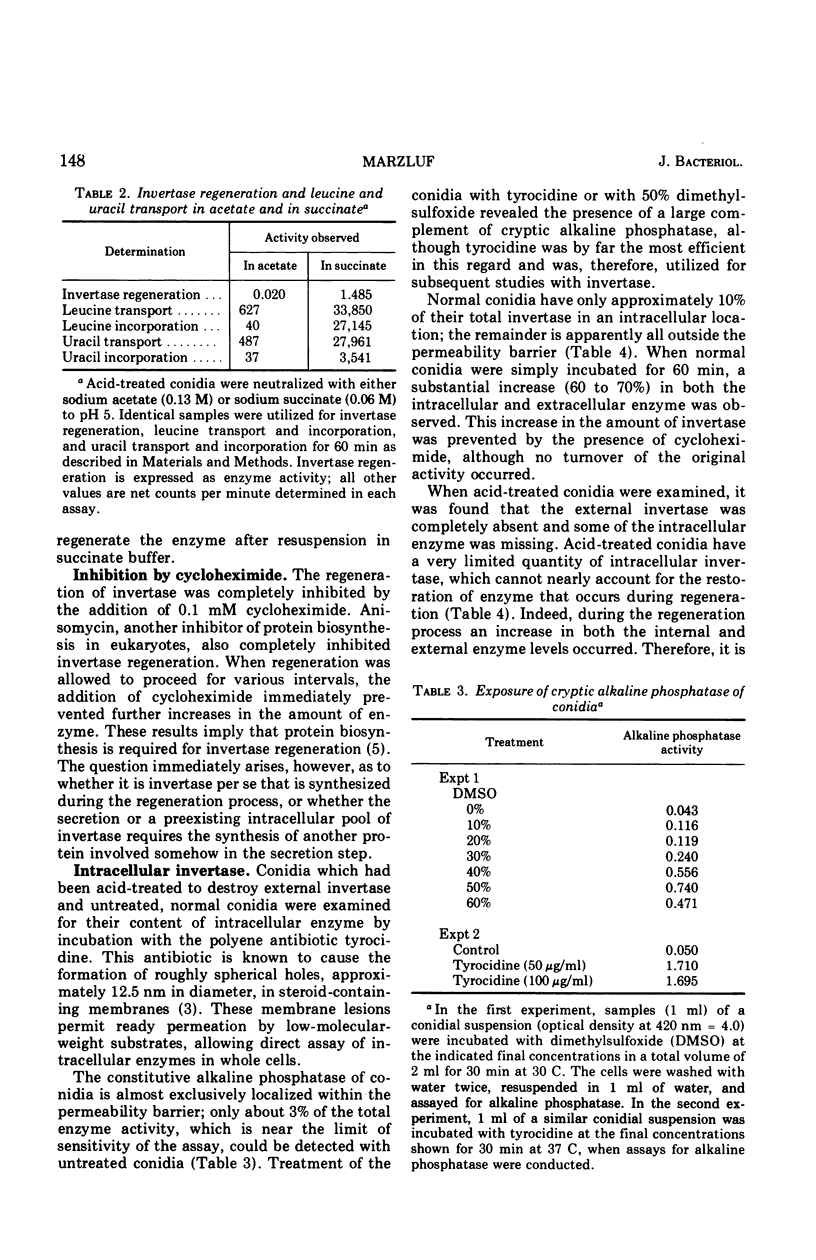
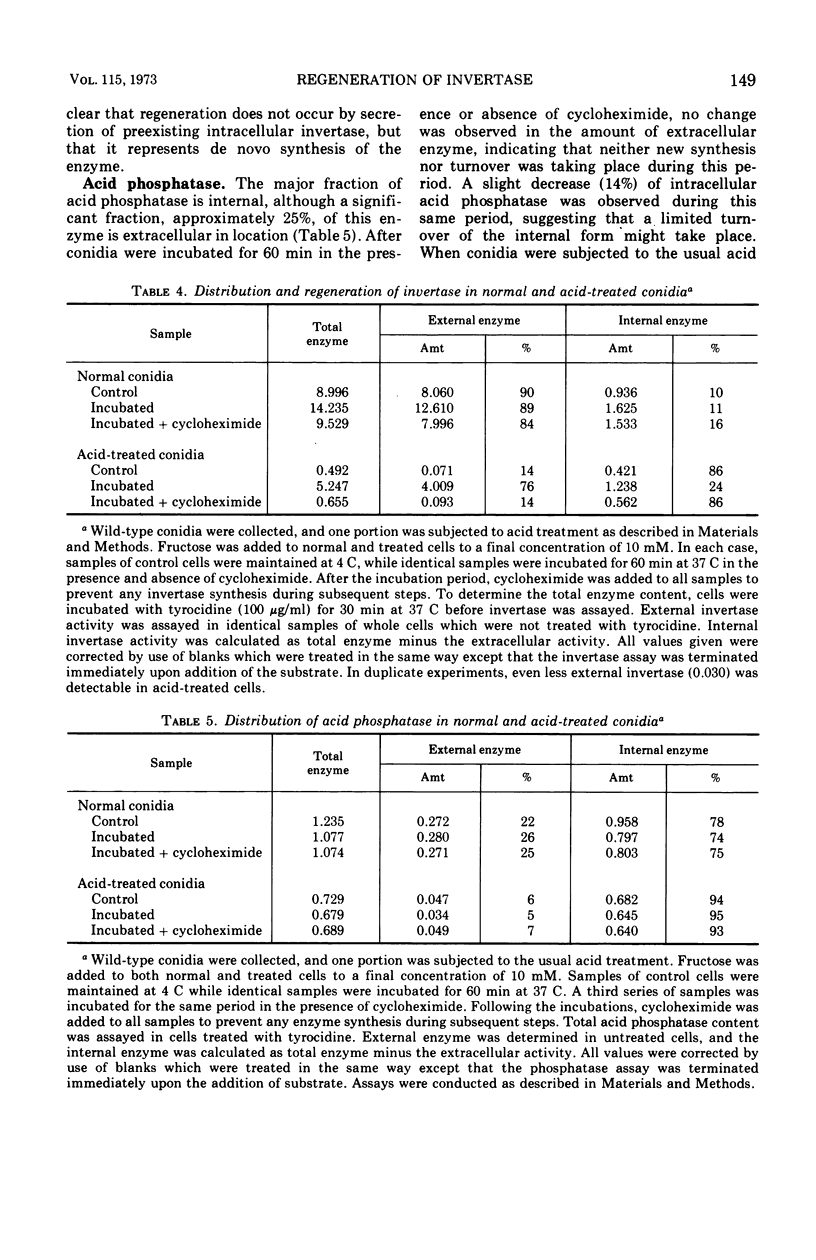
In Neurospora, invertase is predominately an extracellular enzyme, and acid phosphatase is partially external in location. Both extracellular invertase and acid phosphatase were rapidly and quantitatively inactivated by acid treatment (pH 1.3). When such acid-treated cells were incubated with a suitable carbon source, a substantial regeneration of invertase activity occurred, but no restoration of acid phosphatase could be detected. The regeneration of invertase does not occur by renaturation of the inactivated enzyme, nor by secretion of a preexisting intracellular pool of invertase, but instead requires de novo enzyme synthesis. Invertase synthesis was partially repressed by glucose and mannose and was completely inhibited by 2-deoxyglucose. Acetate was found to inhibit invertase regeneration and the transport and incorporation of uracil and leucine. Several potential inhibitors of transcription, including alpha-amanitin, 5-fluorouracil, actinomycin D, and three derivatives of rifamycin, were ineffective in preventing invertase regeneration and in inhibiting the synthesis of ribonucleic acid. Conidia appeared to be very poorly permeable to these compounds.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B. G. Method for decryptification of -glucosidase in yeast with dimethyl sulfoxide. Anal Biochem. 1972 Jan;45(1):137–146. doi: 10.1016/0003-2697(72)90014-0. [DOI] [PubMed] [Google Scholar]
- Adman R., Schultz L. D., Hall B. D. Transcription in yeast: separation and properties of multiple FNA polymerases. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1702–1706. doi: 10.1073/pnas.69.7.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard M. Biochemical and genetic aspects of nystatin resistance in saccharomyces cerevisiae. J Bacteriol. 1972 Sep;111(3):649–657. doi: 10.1128/jb.111.3.649-657.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz N. H., Feldman H. M., Pall M. L. Derepression of tyrosinase synthesis in Neurospora by cycloheximide, actinomycin D, and puromycin. J Biol Chem. 1970 Jun 10;245(11):2784–2788. [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Inhibition by 2-deoxy-D-glucose of synthesis of glycoprotein enzymes by protoplasts of Saccharomyces: relation to inhibition of sugar uptake and metabolism. J Bacteriol. 1972 Aug;111(2):419–429. doi: 10.1128/jb.111.2.419-429.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liras P., Gascón S. Biosynthesis and secretion of yeast invertase. Effect of cycloheximide and 2-deoxy-D-glucose. Eur J Biochem. 1971 Nov 11;23(1):160–165. doi: 10.1111/j.1432-1033.1971.tb01603.x. [DOI] [PubMed] [Google Scholar]
- METZENBERG R. L. A gene affecting the repression of invertase and trehalase in Neurospora. Arch Biochem Biophys. 1962 Mar;96:468–474. doi: 10.1016/0003-9861(62)90322-3. [DOI] [PubMed] [Google Scholar]
- METZENBERG R. L. THE LOCALIZATION OF BETA-FRUCTOFURANOSIDASE IN NEUROSPORA. Biochim Biophys Acta. 1963 Nov 8;77:455–465. doi: 10.1016/0006-3002(63)90521-3. [DOI] [PubMed] [Google Scholar]
- METZENBERG R. L. The purification and properties of invertase of Neurospora. Arch Biochem Biophys. 1963 Mar;100:503–511. doi: 10.1016/0003-9861(63)90118-8. [DOI] [PubMed] [Google Scholar]
- Meachum Z. D., Jr, Colvin H. J., Jr, Braymer H. D. Chemical and physical studies of Neurospora crassa invertase. Molecular weight, amino acid and carbohydrate composition, and quaternary structure. Biochemistry. 1971 Jan 19;10(2):326–332. doi: 10.1021/bi00778a020. [DOI] [PubMed] [Google Scholar]
- Meilhac M., Tysper Z., Chambon P. Animal DNA-dependent RNA polymerases. 4. Studies on inhibition by rifamycin derivatives. Eur J Biochem. 1972 Jul 13;28(2):291–300. doi: 10.1111/j.1432-1033.1972.tb01913.x. [DOI] [PubMed] [Google Scholar]
- Schiltz J. R., Terry K. D. Nucleoside uptake during the germination of Neurospora crassa conidia. Biochim Biophys Acta. 1970;209(2):278–288. doi: 10.1016/0005-2787(70)90726-4. [DOI] [PubMed] [Google Scholar]
- Schneider R. P., Wiley W. R. Transcription and degradation of messenger ribonucleic acid for a glucose transport system in Neurospora. J Biol Chem. 1971 Aug 10;246(15):4784–4789. [PubMed] [Google Scholar]
- Scott W. A., Metzenberg R. L. Location of Aryl Sulfatase in Conidia and Young Mycelia of Neurospora crassa. J Bacteriol. 1970 Dec;104(3):1254–1265. doi: 10.1128/jb.104.3.1254-1265.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu C. W., Konings W. N., Freese E. Effects of acetate and other short-chain fatty acids on sugar and amino acid uptake of Bacillus subtilis. J Bacteriol. 1972 Aug;111(2):525–530. doi: 10.1128/jb.111.2.525-530.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian K. N., Sorger G. J. Regulation of nitrate reductase in Neurospora crassa: regulation of transcription and translation. J Bacteriol. 1972 May;110(2):547–553. doi: 10.1128/jb.110.2.547-553.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale J. H., DeBusk A. G. Developmental regulation of amino acid transport in Neurospora crassa. J Bacteriol. 1970 Nov;104(2):689–697. doi: 10.1128/jb.104.2.689-697.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-E A., Ishikawa T. Genetic control of the synthesis of repressible phosphatases in Neurospora crassa. Genetics. 1971 Nov;69(3):339–351. doi: 10.1093/genetics/69.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totten R. E., Howe H. B., Jr Temperature-dependent actinomycin D effect on RNA synthesis during synchronous development in Neurospora crassa. Biochem Genet. 1971 Dec;5(6):521–532. doi: 10.1007/BF00485670. [DOI] [PubMed] [Google Scholar]
- Turner J. R., Terry K., Matchett W. H. Temporal separation of transcription and translation in Neurospora. J Bacteriol. 1970 Aug;103(2):370–374. doi: 10.1128/jb.103.2.370-374.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterburn P. J., Phelps C. F. The significance of glycosylated proteins. Nature. 1972 Mar 24;236(5343):147–151. doi: 10.1038/236147a0. [DOI] [PubMed] [Google Scholar]


