Abstract
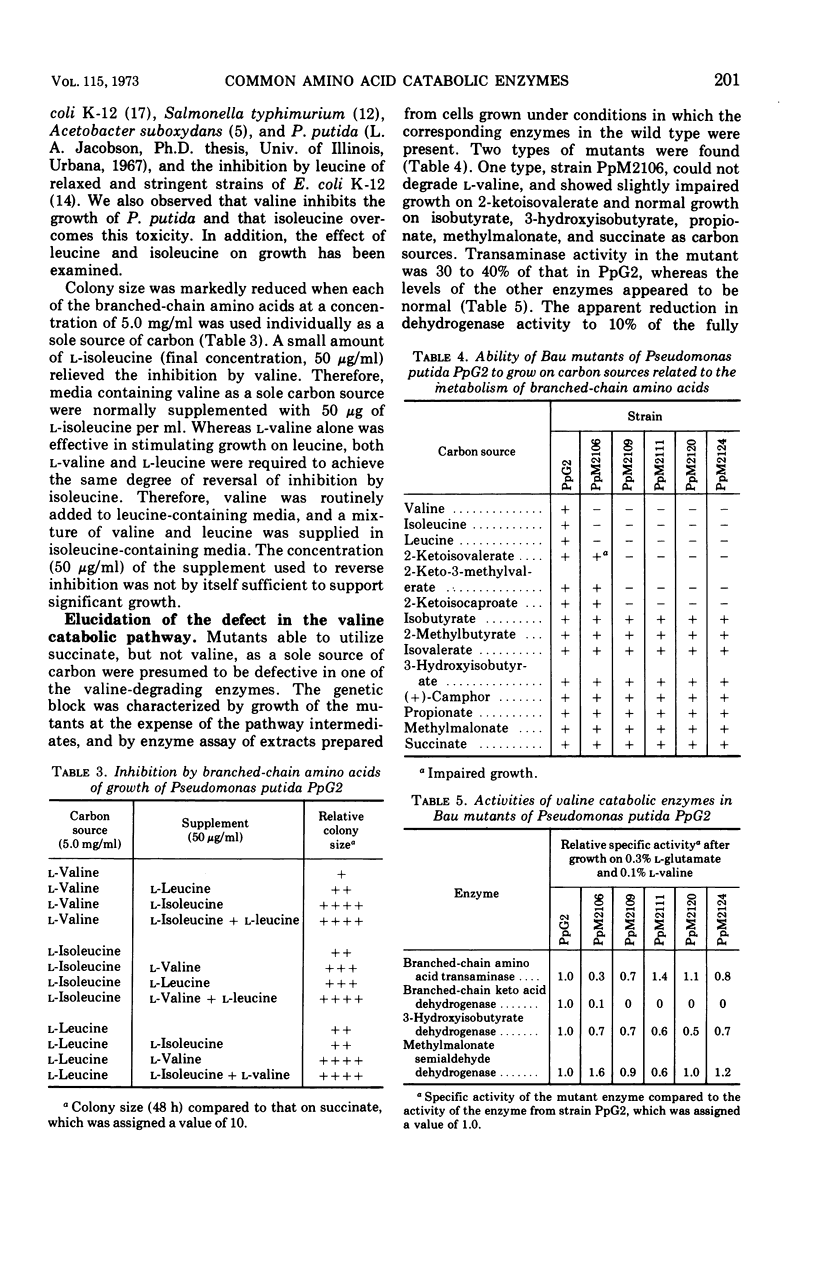
Two types of Pseudomonas putida PpG2 mutants which were unable to degrade branched-chain amino acids were isolated after mutagenesis and selection for ability to grow on succinate, but not valine, as a sole source of carbon. These isolates were characterized by growth on the three branched-chain amino acids (valine, isoleucine, and leucine), on the corresponding branched-chain keto acids (2-ketoisovalerate, 2-keto-3-methylvalerate, and 2-ketoisocaproate), and on other selected intermediates as carbon sources, and by their enzymatic composition. One group of mutants lost 2-ketoisovalerate-inducible branched-chain keto acid dehydrogenase that was active on all three keto acids. There was also a concomitant loss of ability to grow on all three branched-chain amino acids as well as on all three corresponding keto acids, but there was retention of ability to use subsequent intermediates in the catabolism of branched-chain amino acids. Another type of mutant showed a marked reduction in branched-chain amino acid transaminase activity and grew poorly at the expense of all three amino acids, but it utilized subsequent intermediates as carbon sources. Both the transaminase and branched-chain keto acid dehydrogenase mutants retained the ability to degrade camphor. These findings are consistent with the view that branched-chain amino acid transaminase and branched-chain keto acid dehydrogenase are common enzymes in the catabolism of valine, isoleucine, and leucine.
Full text
PDF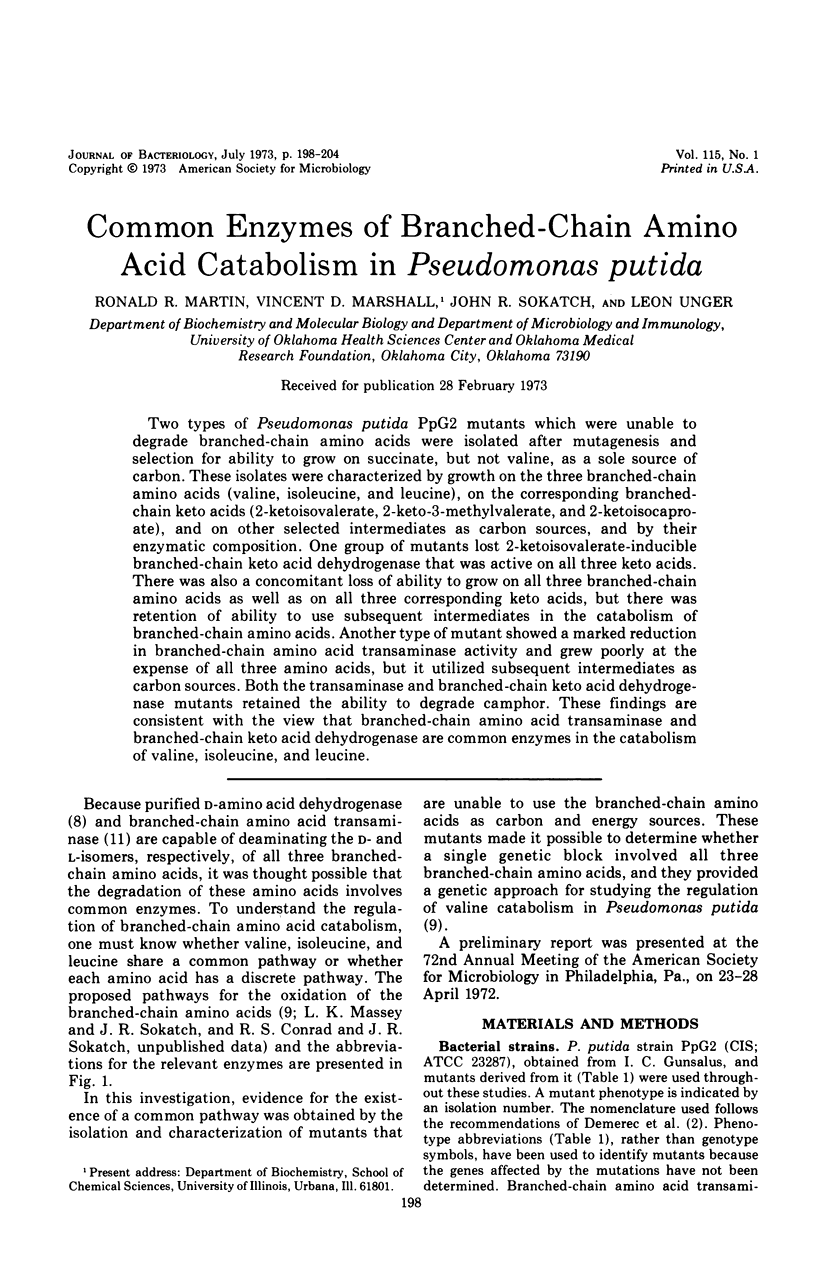




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L. A., Bartholomaus R. C., Gunsalus I. C. Repression of malic enzyme by acetate in Pseudomonas. Biochem Biophys Res Commun. 1966 Sep 22;24(6):955–960. doi: 10.1016/0006-291x(66)90343-3. [DOI] [PubMed] [Google Scholar]
- KERWAR S. S., CHELDELIN V. H., PARKS L. W. VALINE-ISOLEUCINE METABOLISM IN ACETOBACTER SUBOXYDANS AND THE INHIBITION OF GROWTH BY VALINE. J Bacteriol. 1964 Jul;88:179–186. doi: 10.1128/jb.88.1.179-186.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Gronlund A. F. Amino acid transport in Pseudomonas aeruginosa. J Bacteriol. 1969 Jan;97(1):273–281. doi: 10.1128/jb.97.1.273-281.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVELESS A., HOWARTH S. Mutation of bacteria at high levels of survival by ethyl methane sulphonate. Nature. 1959 Dec 5;184:1780–1782. doi: 10.1038/1841780a0. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Loutit J. S. Regulation of isoleucine-valine biosynthesis in Pseudomonas aeruginosa. I. Characterisation and mapping of mutants. Genetics. 1969 Nov;63(3):547–556. doi: 10.1093/genetics/63.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall V. D., Sokatch J. R. Regulation of valine catabolism in Pseudomonas putida. J Bacteriol. 1972 Jun;110(3):1073–1081. doi: 10.1128/jb.110.3.1073-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall V. P., Sokatch J. R. Oxidation of D-amino acids by a particulate enzyme from Pseudomonas aeruginosa. J Bacteriol. 1968 Apr;95(4):1419–1424. doi: 10.1128/jb.95.4.1419-1424.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTON J. E., BULMER G. S., SOKATCH J. R. THE OXIDATION OF D-ALANINE BY CELL MEMBRANES OF PSEUDOMONAS AERUGINOSA. Biochim Biophys Acta. 1963 Oct 8;78:136–147. doi: 10.1016/0006-3002(63)91619-6. [DOI] [PubMed] [Google Scholar]
- Norton J. E., Sokatch J. R. Purification and partial characterization of the branched chain amino acid transaminase of Pseudomonas aeruginosa. Biochim Biophys Acta. 1970 May 13;206(2):261–269. doi: 10.1016/0005-2744(70)90109-9. [DOI] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. I. GENETIC DEREPRESSION OF ENZYME FORMATION. J Bacteriol. 1964 Mar;87:566–573. doi: 10.1128/jb.87.3.566-573.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON W. G., COON M. J. The purification and properties of beta-hydroxyisobutyric dehydrogenase. J Biol Chem. 1957 Mar;225(1):511–521. [PubMed] [Google Scholar]
- Rogerson A. C., Freundlich M. Control of isoleucine, valine and leucine biosynthesis. 8. Mechanism of growth inhibition by leucine in relaxed and stringent strains of Escherichia coli K-12. Biochim Biophys Acta. 1970 Apr 14;208(1):87–98. doi: 10.1016/0304-4165(70)90051-6. [DOI] [PubMed] [Google Scholar]
- Sokatch J. R., Sanders L. E., Marshall V. P. Oxidation of methylmalonate semialdehyde to propionyl coenzyme A in Pseudomonas aeruginosa grown on valine. J Biol Chem. 1968 May 25;243(10):2500–2506. [PubMed] [Google Scholar]
- Taylor R. T., Jenkins W. T. Leucine aminotransferase. I. Colorimetric assays. J Biol Chem. 1966 Oct 10;241(19):4391–4395. [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Isoleucine and valine metabolism in Escherichia coli. V. Antagonism between isoleucine and valine. J Bacteriol. 1955 Aug;70(2):241–248. doi: 10.1128/jb.70.2.241-248.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


