Abstract
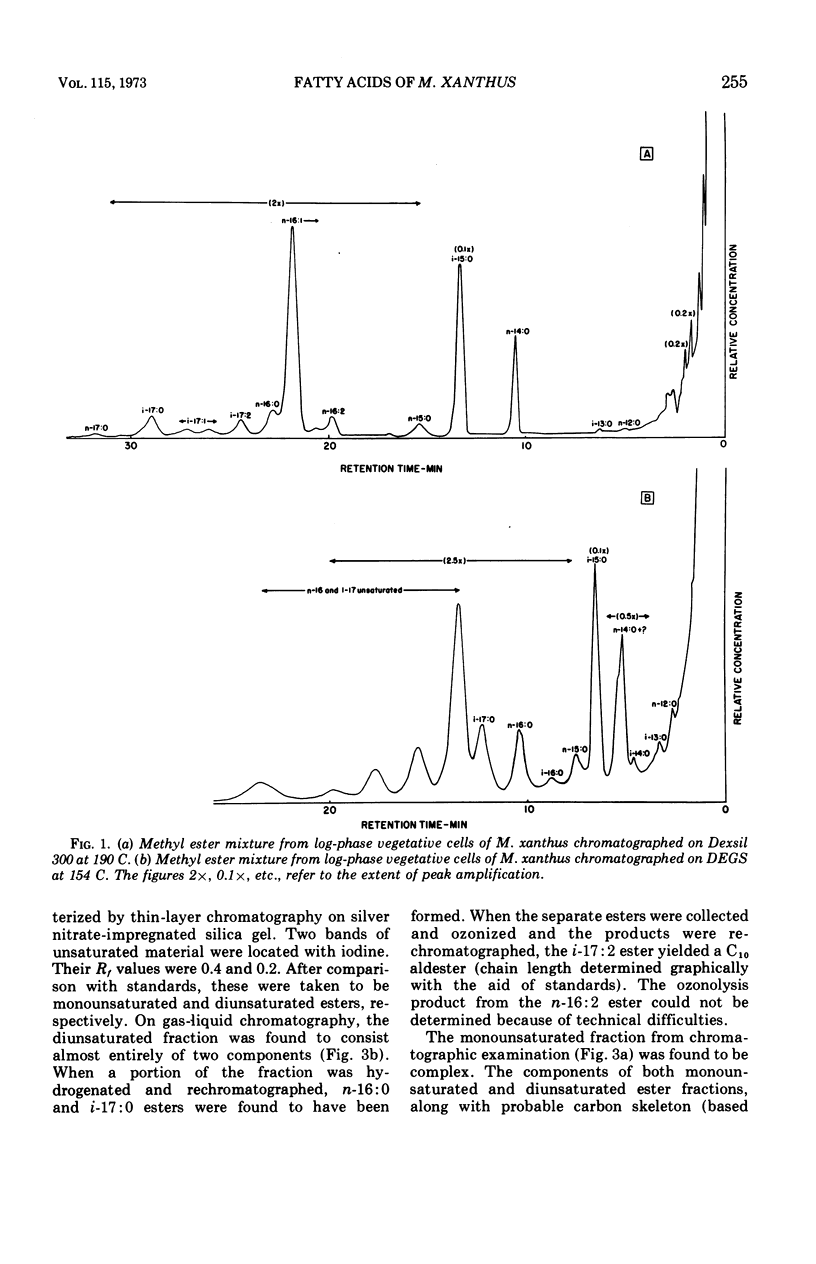
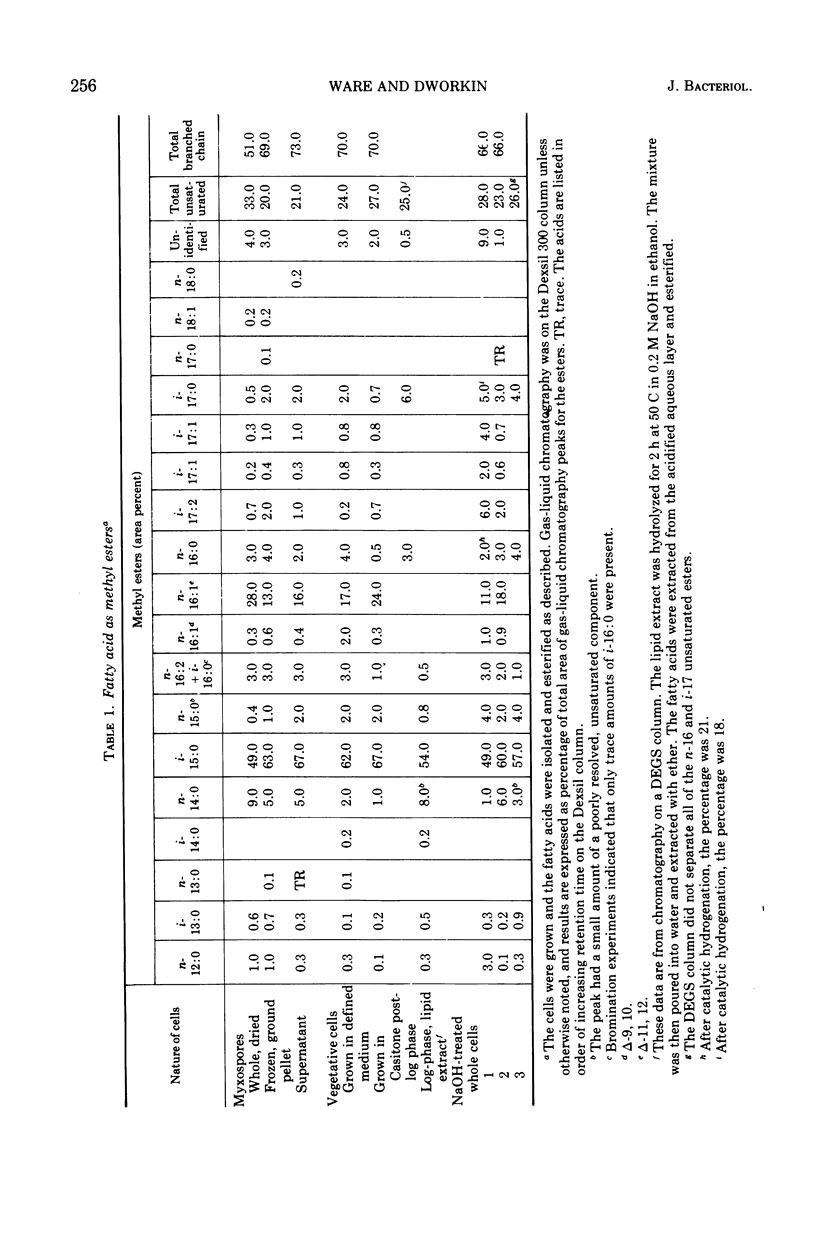
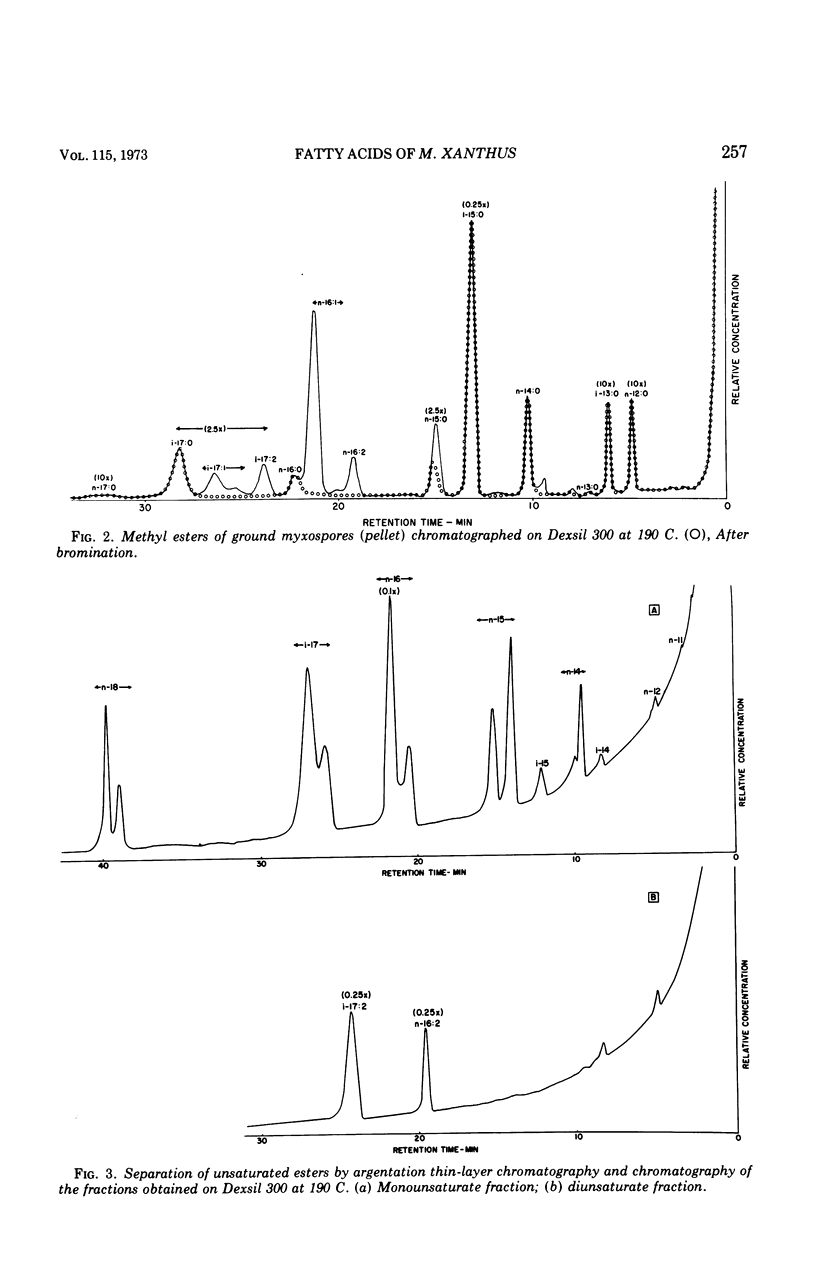
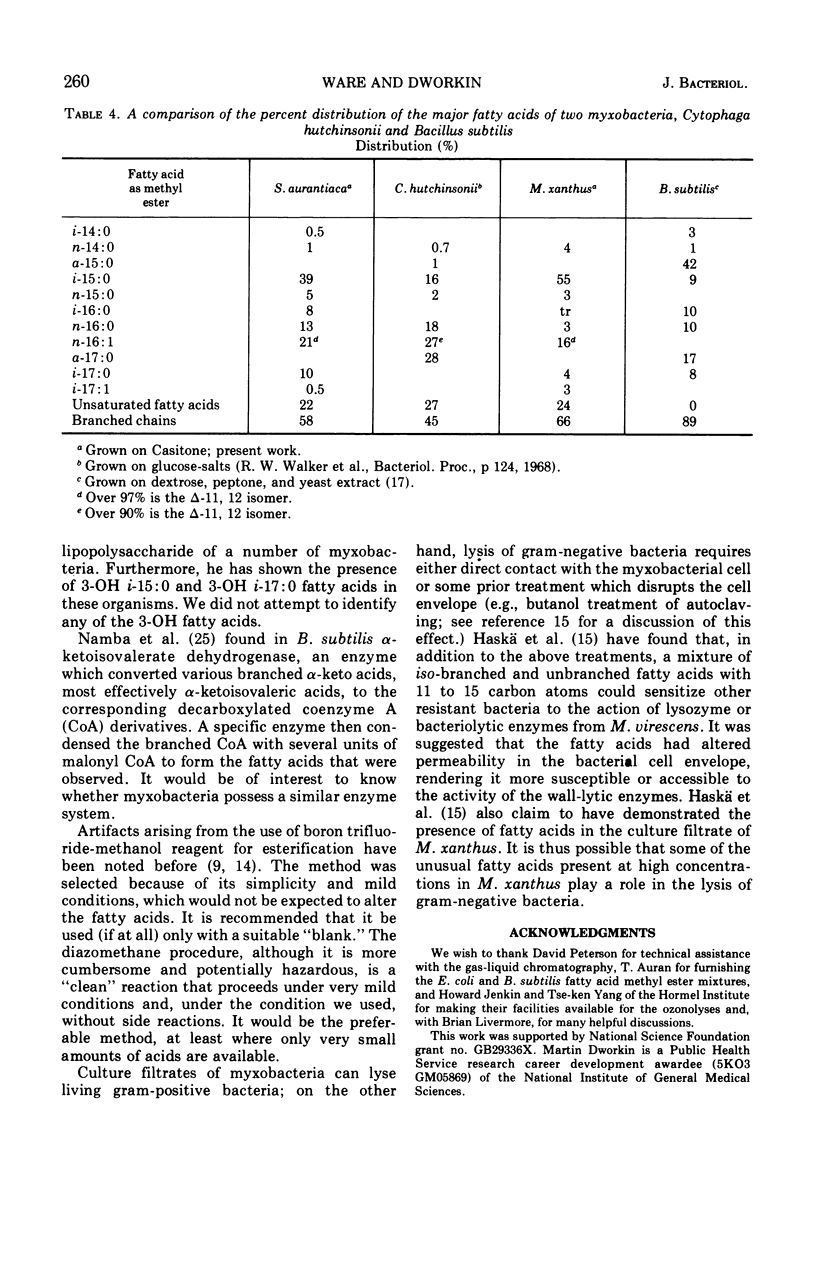
Fatty acids were extracted from saponified vegetative cells and myxospores of Myxococcus xanthus and examined as the methyl esters by gas-liquid chromatography. The acids consisted mainly of C14 to C17 species. Branched acids predominated, and iso-pentadecanoic acid constituted half or more of the mixture. The other leading component (11–28%) was found to be 11-n-hexadecenoic acid. Among the unsaturated acids were two diunsaturated ones, an n-hexadecadienoic acid and an iso-heptadecadienoic acid. No significant differences between the fatty acid compositions of the vegetative cells and myxospores could be detected. The fatty acid composition of M. xanthus was found to be markedly similar to that of Stigmatella aurantiaca. It is suggested that a fatty acid pattern consisting of a large proportion of iso-branched C15 and C17 acids and a substantial amount of an n-16:1 acid is characteristic of myxobacteria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON M. J., BRYANT M. P., KATZ I., KEENEY M. Metabolic function of branched-chain volatile fatty acids, growth factors for ruminococci. II. Biosynthesis of higher branched-chain fatty acids and aldehydes. J Bacteriol. 1962 May;83:1084–1093. doi: 10.1128/jb.83.5.1084-1093.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman A. J., Simmonds P. G. Fatty acids and polar lipids of extremely thermophilic filamentous bacterial masses from two Yellowstone hot springs. J Bacteriol. 1969 May;98(2):528–531. doi: 10.1128/jb.98.2.528-531.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. Y., Salton M. R. Fatty acid composition of bacterial membrane and wall lipids. Biochim Biophys Acta. 1966 Feb 1;116(1):73–79. doi: 10.1016/0005-2760(66)90093-2. [DOI] [PubMed] [Google Scholar]
- DWORKIN M., GIBSON S. M. A SYSTEM FOR STUDYING MICROBIAL MORPHOGENESIS: RAPID FORMATION OF MICROCYSTS IN MYXOCOCCUS XANTHUS. Science. 1964 Oct 9;146(3641):243–244. doi: 10.1126/science.146.3641.243. [DOI] [PubMed] [Google Scholar]
- DWORKIN M. Nutritional requirements for vegetative growth of Myxococcus xanthus. J Bacteriol. 1962 Aug;84:250–257. doi: 10.1128/jb.84.2.250-257.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawidowicz E. A., Thompson T. E. Artifacts produced by boron trifluoride methanolysis of a synthetic lecithin containing cyclopropane fatty acids (1-2-dihydrosterculoyl-3-sn-phosphatidylcholine). J Lipid Res. 1971 Sep;12(5):636–637. [PubMed] [Google Scholar]
- Fulco A. J. Bacterial biosynthesis of polyunsaturated fatty acids. Biochim Biophys Acta. 1969 Jul 29;187(1):169–171. doi: 10.1016/0005-2760(69)90149-0. [DOI] [PubMed] [Google Scholar]
- Fulk W. K., Shorb M. S. Production of an artifact during methanolysis of lipids by boron trifluoride-methanol. J Lipid Res. 1970 May;11(3):276–277. [PubMed] [Google Scholar]
- Hemphill H. E., Zahler S. A. Nutritional induction and suppression of fruiting in Myxococcus xanthus FBa. J Bacteriol. 1968 Mar;95(3):1018–1023. doi: 10.1128/jb.95.3.1018-1023.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Biosynthesis of branched-chain fatty acids. IV. Factors affecting relative abundance of fatty acids produced by Bacillus subtilis. Can J Microbiol. 1966 Jun;12(3):501–514. doi: 10.1139/m66-073. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids in Bacillus larvae, Bacillus lentimorbus, and Bacillus popilliae. J Bacteriol. 1969 Apr;98(1):143–146. doi: 10.1128/jb.98.1.143-146.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids in the genus Bacillus. I. Iso- and anteiso-fatty acids as characteristic constituents of lipids in 10 species. J Bacteriol. 1967 Mar;93(3):894–903. doi: 10.1128/jb.93.3.894-903.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids in the genus Bacillus. II. Similarity in the fatty acid compositions of Bacillus thuringiensis, Bacillus anthracis, and Bacillus cereus. J Bacteriol. 1968 Jun;95(6):2210–2216. doi: 10.1128/jb.95.6.2210-2216.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo E., Ueta N. Composition of fatty acids and carbohydrates in Leptospira. J Bacteriol. 1972 May;110(2):459–467. doi: 10.1128/jb.110.2.459-467.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba Y., Yoshizawa K., Ejima A., Hayashi T., Kaneda T. Coenzyme A- and nicotinamide adenine dinucleotide-dependent branched chain alpha-keto acid dehydrogenase. I. Purification and properties of the enzyme from Bacillus subtilis. J Biol Chem. 1969 Aug 25;244(16):4437–4447. [PubMed] [Google Scholar]
- SAND D., SEN N., SCHLENK H. POSITIONAL ISOMERISM OF UNSATURATED FATTY ACIDS IN THE RAT. QUANTIFICATION OF ISOMERIC MIXTURES. J Am Oil Chem Soc. 1965 Jun;42:511–516. doi: 10.1007/BF02540093. [DOI] [PubMed] [Google Scholar]
- Sudo S. Z., Dworkin M. Resistance of vegetative cells and microcysts of Myxococcus xanthus. J Bacteriol. 1969 Jun;98(3):883–887. doi: 10.1128/jb.98.3.883-887.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. W. Cis-11-hexadecenoic acid from Cytophaga hutchinsonii lipids. Lipids. 1969 Jan;4(1):15–18. doi: 10.1007/BF02531788. [DOI] [PubMed] [Google Scholar]


