Abstract
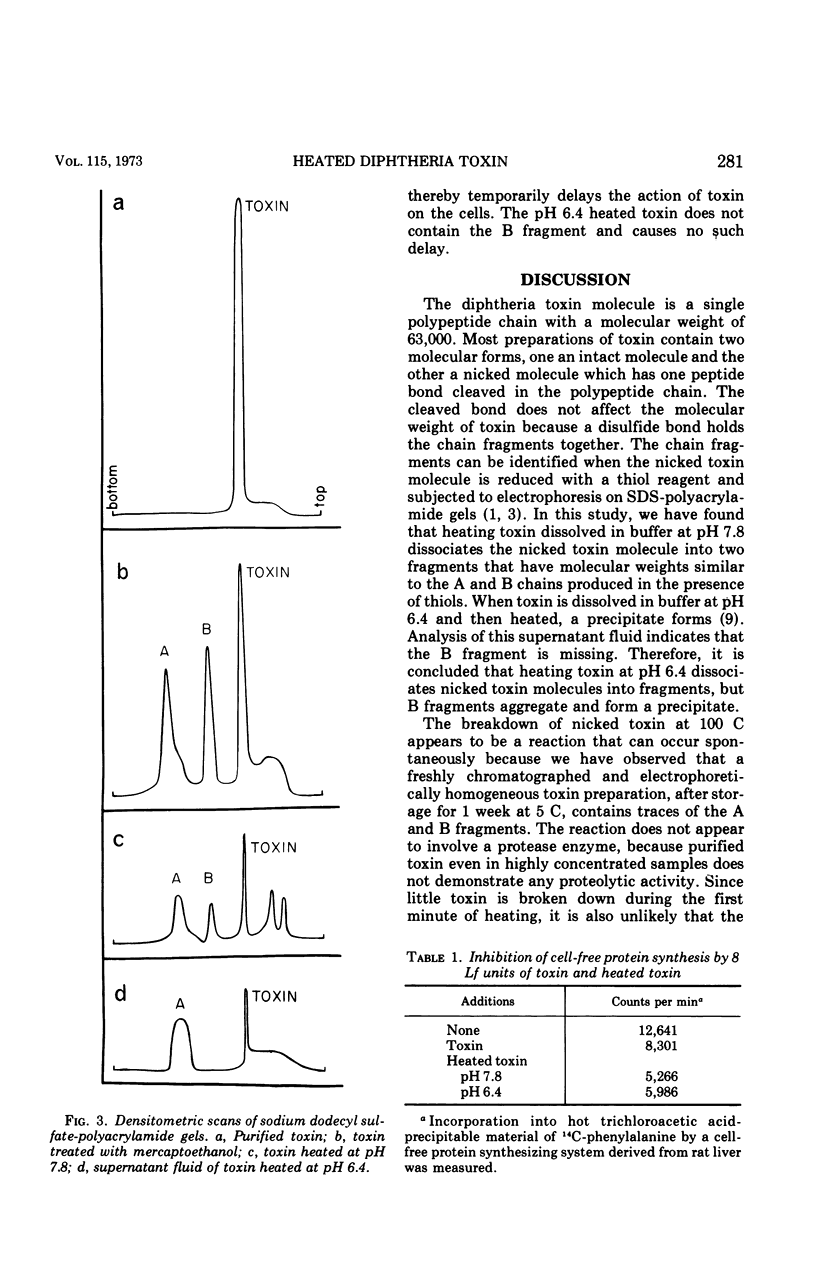
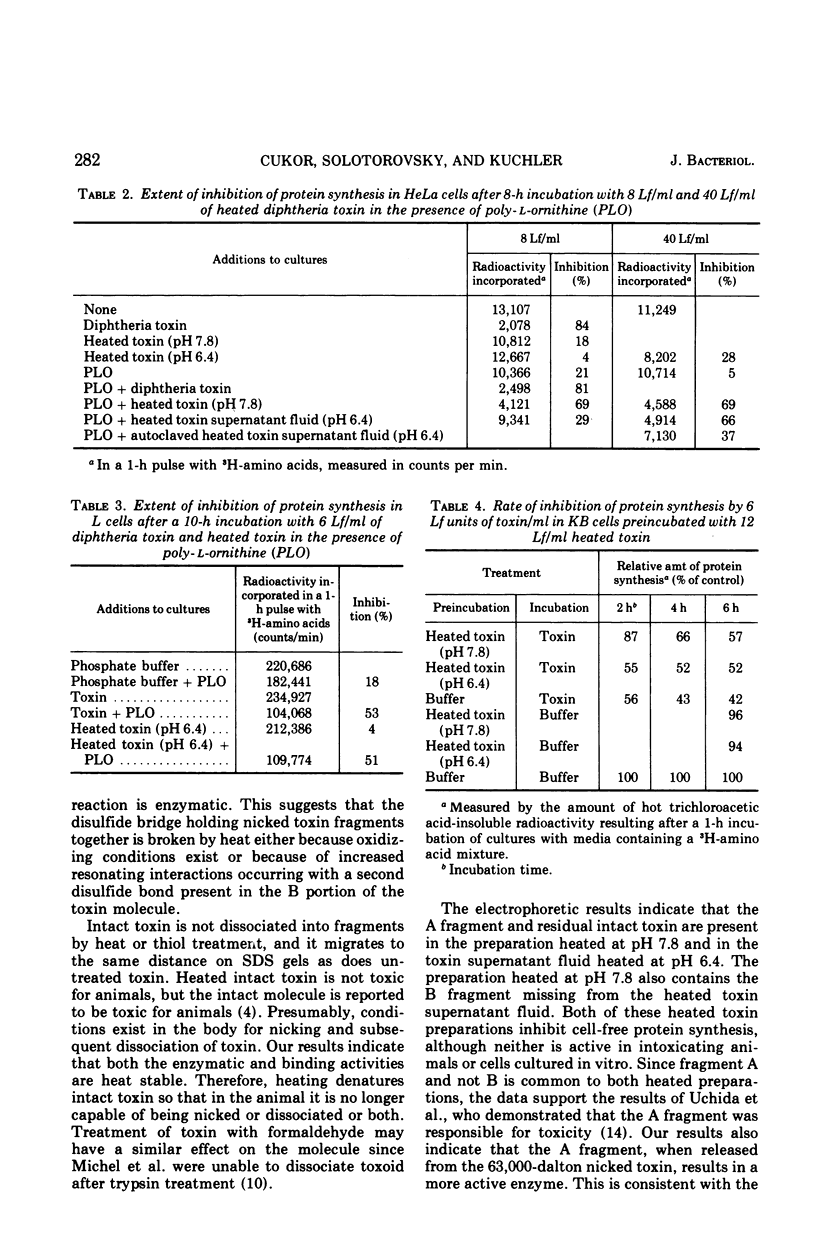
Diphtheria toxin splits into two fragments when heated at 100 C for 10 min in a phosphate buffer. The separated fragments have molecular weights of 24,000 and 39,000, respectively. These molecular weights are similar to those of the A and B fragments found in diphtheria toxin preparations after thiol reduction. Since the separation of toxin into fragments is not complete, it is likely that only nicked toxin molecules having a cleaved peptide bond are split by heating. When toxin is suspended in phosphate buffer at pH 6.4, the B-like fragment precipitates, but at pH 7.8 it does not. Heated toxin is unable to intoxicate sensitive cells or cause a necrodermal response in animals. Fragment A produced by heating is active in inhibiting cell-free protein synthesis. It is able to intoxicate both HeLa and L cells when the uptake of the fragment is facilitated by addition of polyornithine to the cultures. Fragment B produced by heating is involved with binding to the cell surface. It is able to delay the action of toxin on KB cell cultures preincubated with fragment B.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- Drazin R., Kandel J., Collier R. J. Structure and activity of diphtheria toxin. II. Attack by trypsin at a specific site within the intact toxin molecule. J Biol Chem. 1971 Mar 10;246(5):1504–1510. [PubMed] [Google Scholar]
- Gill D. M., Dinius L. L. Observations on the structure of diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1485–1491. [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr Structure-activity relationships in diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1492–1495. [PubMed] [Google Scholar]
- HATA H. Studies on Corynebacterium diphtheriae. V. The attitude of diphtheria bacillus to some growth factors (calcium pantothenate, glutamic acid and tryptophane). Kitasato Arch Exp Med. 1951 Mar;23(4):75-84; English extract, 1-2. [PubMed] [Google Scholar]
- Honjo T., Nishizuka Y., Hayaishi O. Diphtheria toxin-dependent adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis. J Biol Chem. 1968 Jun 25;243(12):3553–3555. [PubMed] [Google Scholar]
- Honjo T., Nishizuka Y., Kato I., Hayaishi O. Adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis by diphtheria toxin. J Biol Chem. 1971 Jul 10;246(13):4251–4260. [PubMed] [Google Scholar]
- Johnson W., Kuchler R. J., Solotorovsky M. Site in cell-free protein synthesis sensitive to diphtheria toxin. J Bacteriol. 1968 Oct;96(4):1089–1098. doi: 10.1128/jb.96.4.1089-1098.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATO I., NAKAMURA H., UCHIDA T., KOYAMA J., KATSURA T. Purification of diphtheria toxin. II. The isolation of crystalline toxin-protein and some of its properties. Jpn J Exp Med. 1960 Apr;30:129–145. [PubMed] [Google Scholar]
- Michel A., Zanen J., Monier C., Crispeels C., Dirkx J. Partial characterization of diphtheria toxin and its subunits. Biochim Biophys Acta. 1972 Feb 29;257(2):249–256. doi: 10.1016/0005-2795(72)90276-0. [DOI] [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J. The response of cultured mammalian cells to diphtheria toxin. II. The resistant cell: enhancement of toxin action by poly-L-ornithine. J Exp Med. 1968 Mar 1;127(3):541–554. doi: 10.1084/jem.127.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Uchida T., Harper A. A. An immunological study of the diphtheria toxin molecule. Immunochemistry. 1972 Sep;9(9):891–906. doi: 10.1016/0019-2791(72)90163-2. [DOI] [PubMed] [Google Scholar]
- Savage H. E., Kuchler R. J. A versatile composite gel column for the electrophoretic separation of ribonucleic acid molecules. Prep Biochem. 1971;1(4):345–360. doi: 10.1080/00327487108081949. [DOI] [PubMed] [Google Scholar]
- Uchida T., Pappenheimer A. M., Jr, Harper A. A. Reconstitution of diphtheria toxin from two nontoxic cross-reacting mutant proteins. Science. 1972 Feb 25;175(4024):901–903. doi: 10.1126/science.175.4024.901. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]