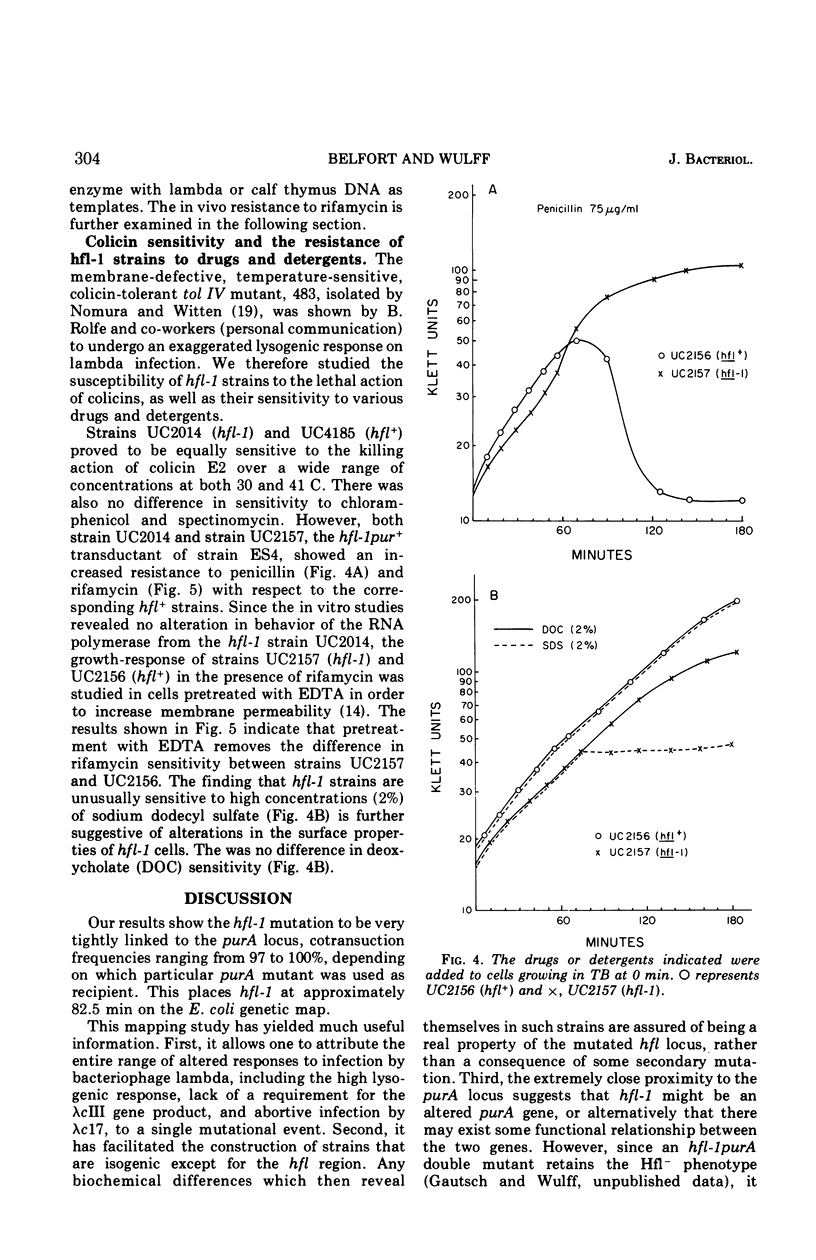
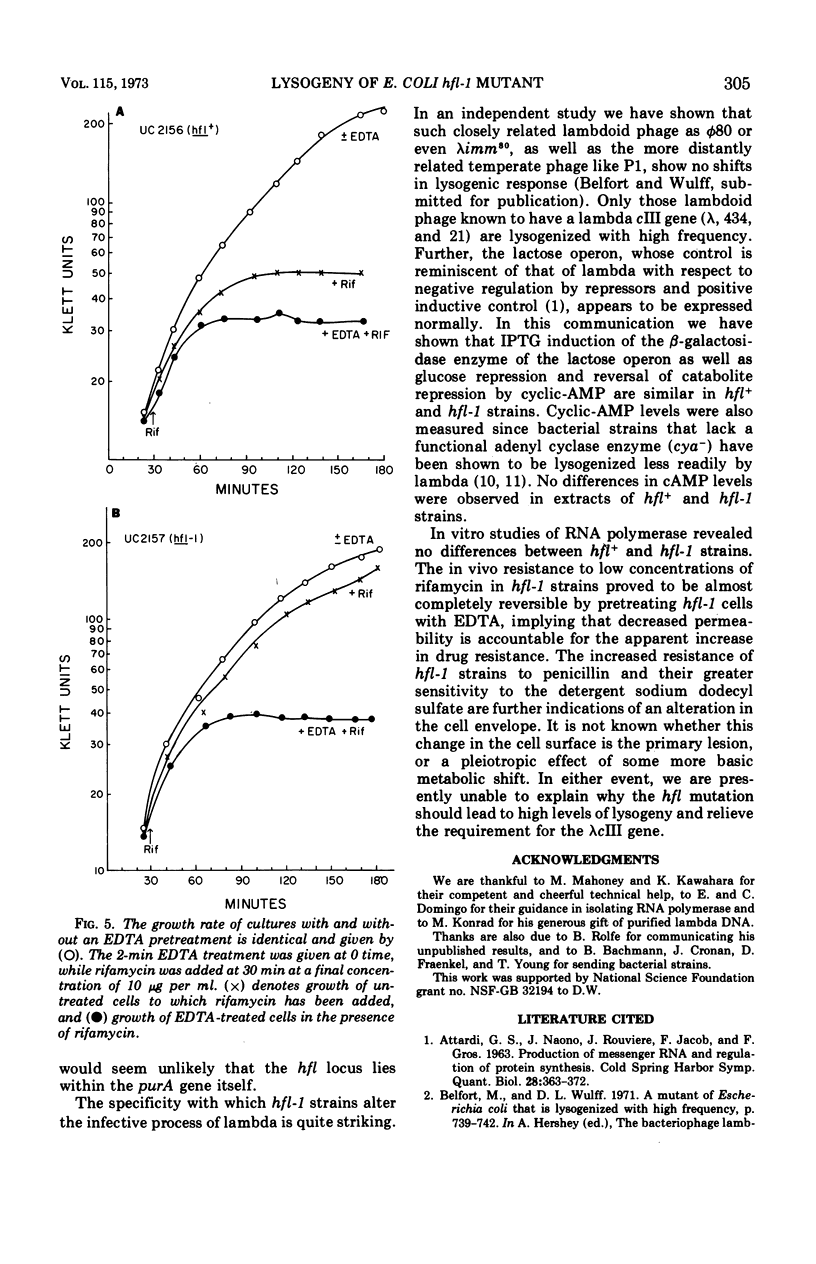
Abstract
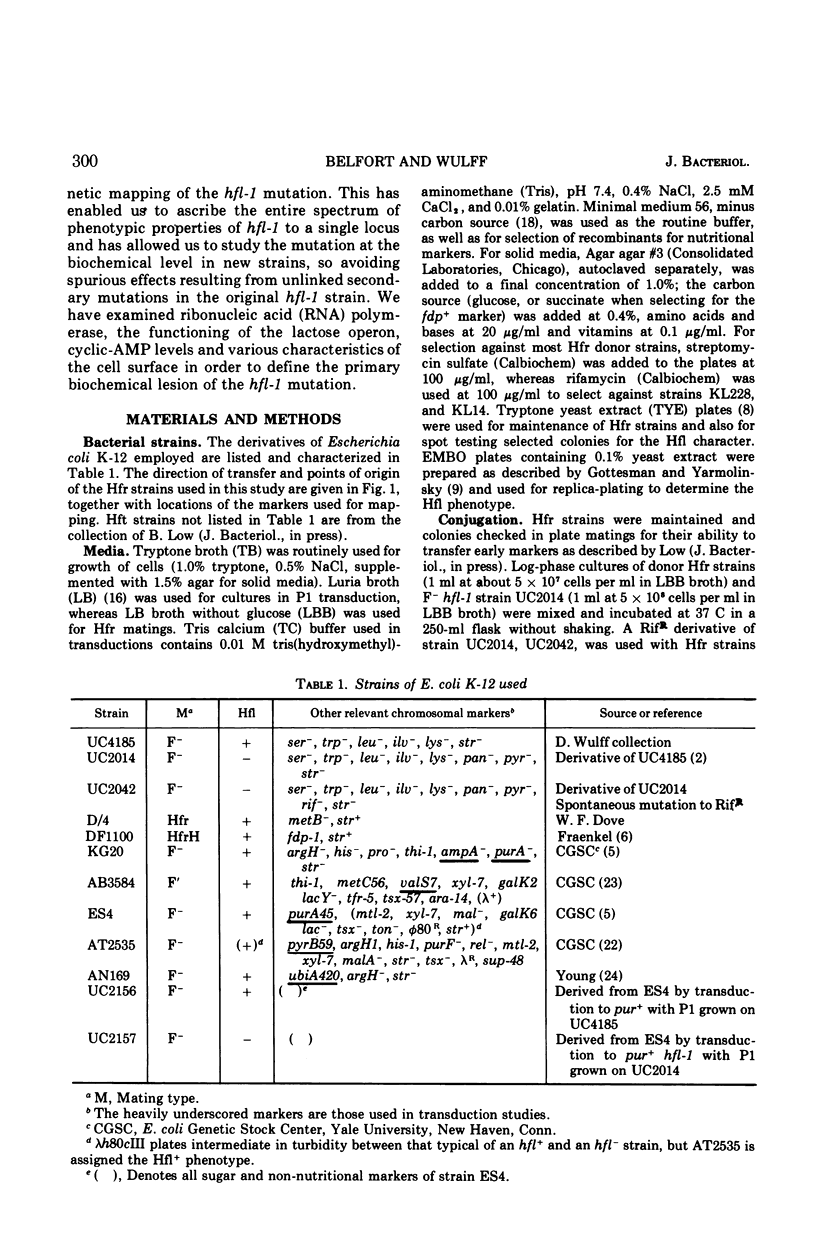
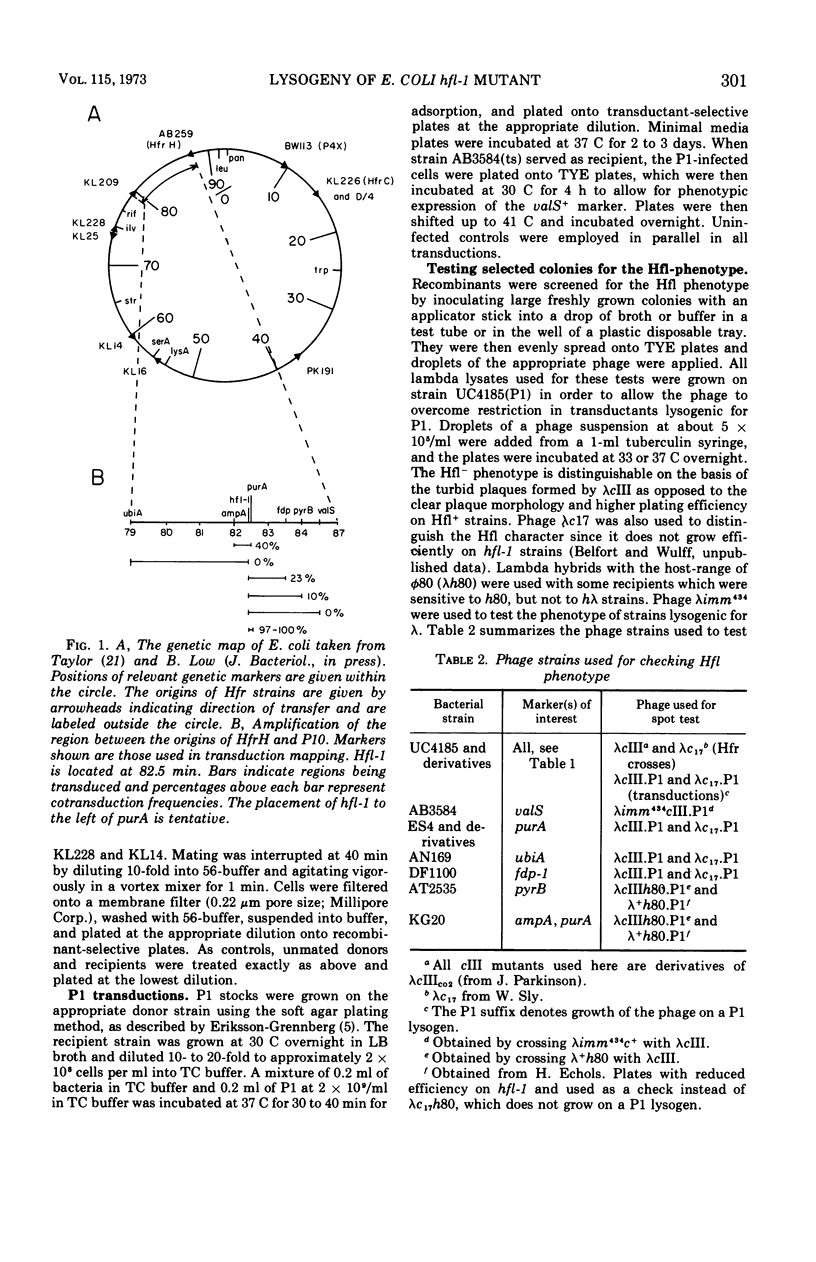
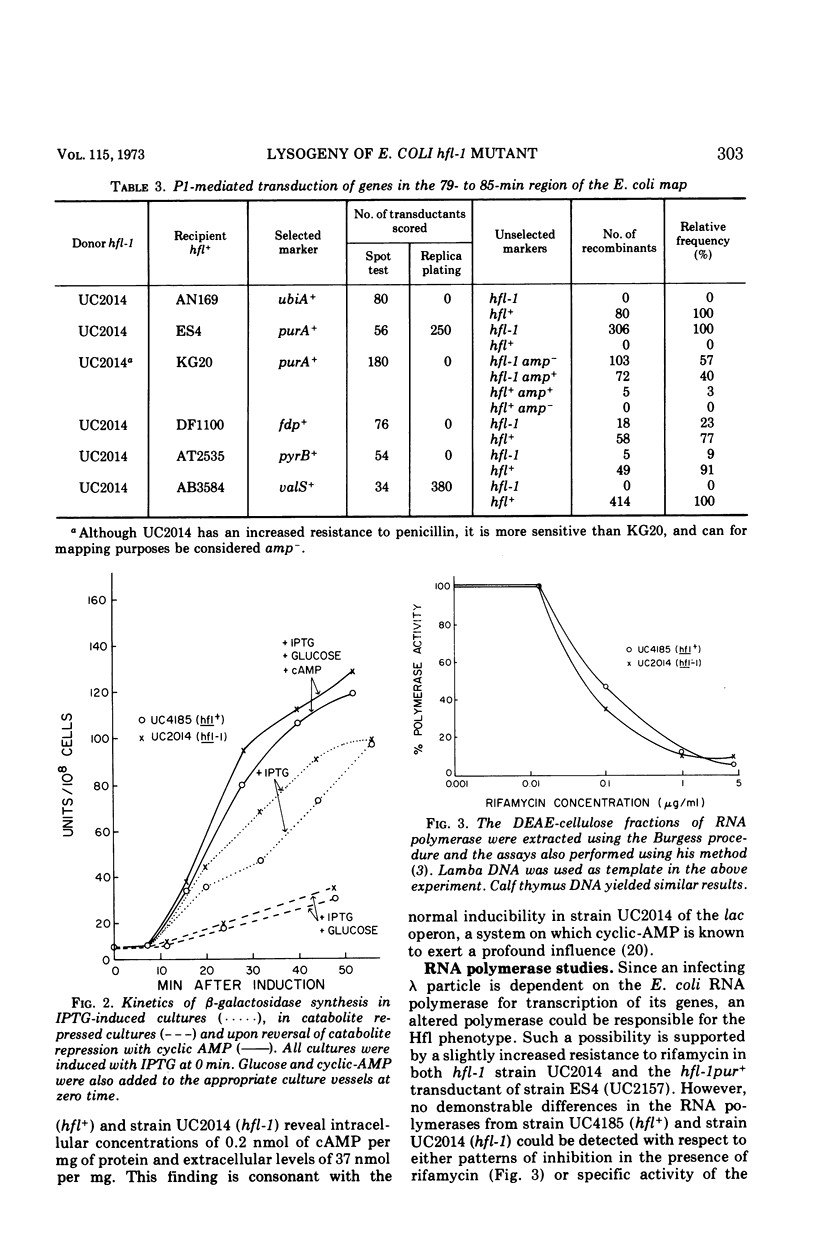
The Escherichia coli mutant hfl-1 is lysogenized at very high frequency by bacteriophage lambda. The normal requirement for the λcIII gene product in the establishment of repression is not observed in hfl-1 strains. These phenotypic characteristics are specified by a single locus at 82.5 min on the E. coli map in extremely close proximity to the purA gene, cotransduction frequencies ranging from 97 to 100% depending on the particular purA marker used. The lactose operon is shown to function normally in this strain, and there are also no demonstrable differences in ribonucleic acid polymerase activity or cyclic-adenosine monophosphate levels. Alterations in the cell envelope are indicated by a slight rifamycin resistance, which is reversible by pretreating the cells with ethylenediaminetetraacetic acid, and by a resistance to penicillin and a sensitivity to high concentrations of sodium dodecyl sulfate. It is not known whether this change in cell surface is the primary lesion, or a pleiotropic effect of some more basic metabolic shift.
Full text
PDF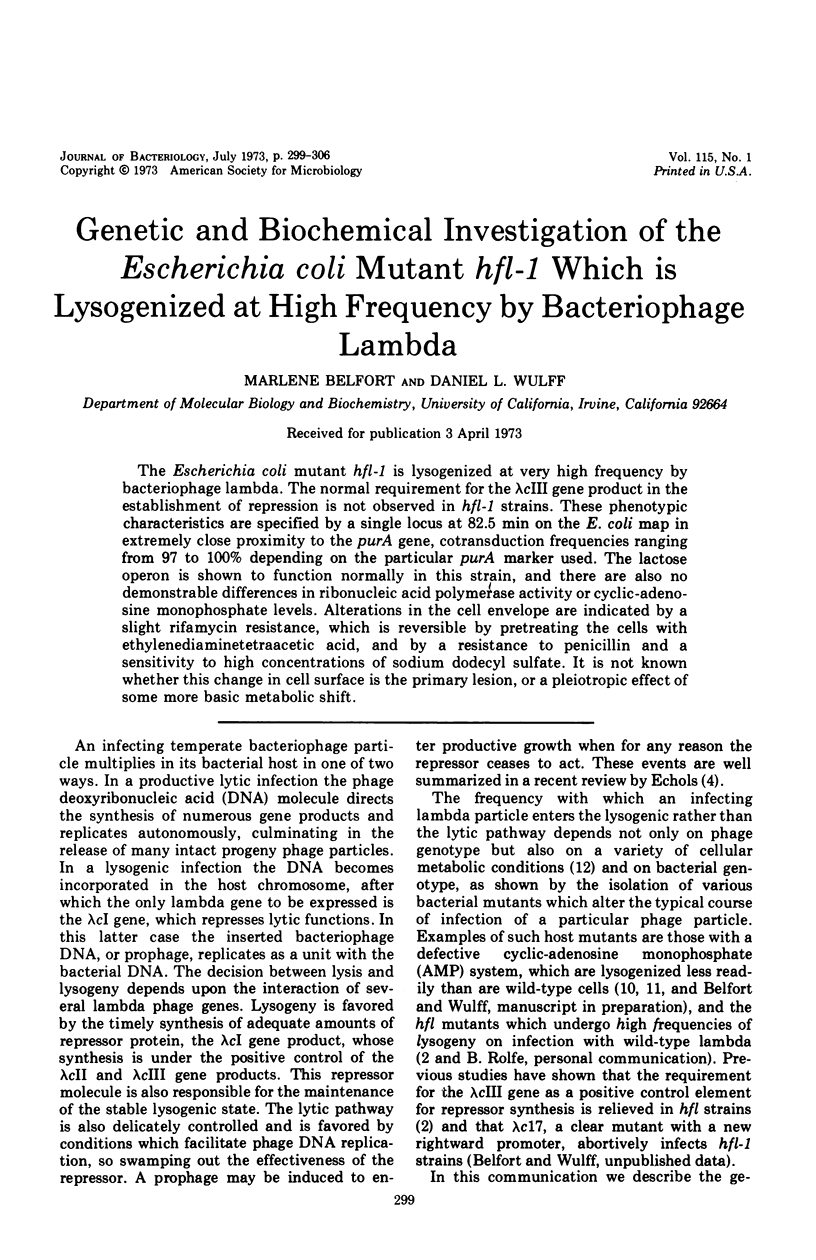


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Echols H. Developmental pathways for the temperate phage: lysis vs lysogeny,. Annu Rev Genet. 1972;6(0):157–190. doi: 10.1146/annurev.ge.06.120172.001105. [DOI] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G. Resistance of Escherichia coli to penicillins. II. An improved mapping of the ampA gene. Genet Res. 1968 Oct;12(2):147–156. doi: 10.1017/s0016672300011769. [DOI] [PubMed] [Google Scholar]
- Fox C. F., Carter J. R., Kennedy E. P. GENETIC CONTROL OF THE MEMBRANE PROTEIN COMPONENT OF THE LACTOSE TRANSPORT SYSTEM OF Escherichia coli. Proc Natl Acad Sci U S A. 1967 Mar;57(3):698–705. doi: 10.1073/pnas.57.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. Integration-negative mutants of bacteriophage lambda. J Mol Biol. 1968 Feb 14;31(3):487–505. doi: 10.1016/0022-2836(68)90423-3. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Beckwith J. R. Directed transposition of the arabinose operon: a technique for the isolation of specialized transducing bacteriophages for any Escherichia coli gene. J Mol Biol. 1969 Aug 28;44(1):117–127. doi: 10.1016/0022-2836(69)90408-2. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Arditti R. R., Eisen H. Establishment of repression by lambdoid phage in catabolite activator protein and adenylate cyclase mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Feb;69(2):366–370. doi: 10.1073/pnas.69.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Smith G. R., Ames B. N. Adenosine 3':5'-cyclic monophosphate concentration in the bacterial host regulates the viral decision between lysogeny and lysis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2258–2262. doi: 10.1073/pnas.68.9.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNELL D., MAGASANIK B. THE CONTROL OF THE RATE OF ENZYME SYNTHESIS IN AEROBACTER AEROGENES. Biochim Biophys Acta. 1964 Mar 9;81:418–434. doi: 10.1016/0926-6569(64)90127-0. [DOI] [PubMed] [Google Scholar]
- LEIVE L. ACTINOMYCIN SENSITIVITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Biochem Biophys Res Commun. 1965 Jan 4;18:13–17. doi: 10.1016/0006-291x(65)90874-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Nomura M., Witten C. Interaction of colicins with bacterial cells. 3. Colicin-tolerant mutations in Escherichia coli. J Bacteriol. 1967 Oct;94(4):1093–1111. doi: 10.1128/jb.94.4.1093-1111.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingle M. A., Neidhardt F. C. Mapping of a structural gene for valyl-transfer ribonucleic acid synthetase in Escherichia coli by transduction. J Bacteriol. 1969 May;98(2):837–839. doi: 10.1128/jb.98.2.837-839.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. G., Leppik R. A., Hamilton J. A., Gibson F. Biochemical and genetic studies on ubiquinone biosynthesis in Escherichia coli K-12:4-hydroxybenzoate octaprenyltransferase. J Bacteriol. 1972 Apr;110(1):18–25. doi: 10.1128/jb.110.1.18-25.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


