Abstract
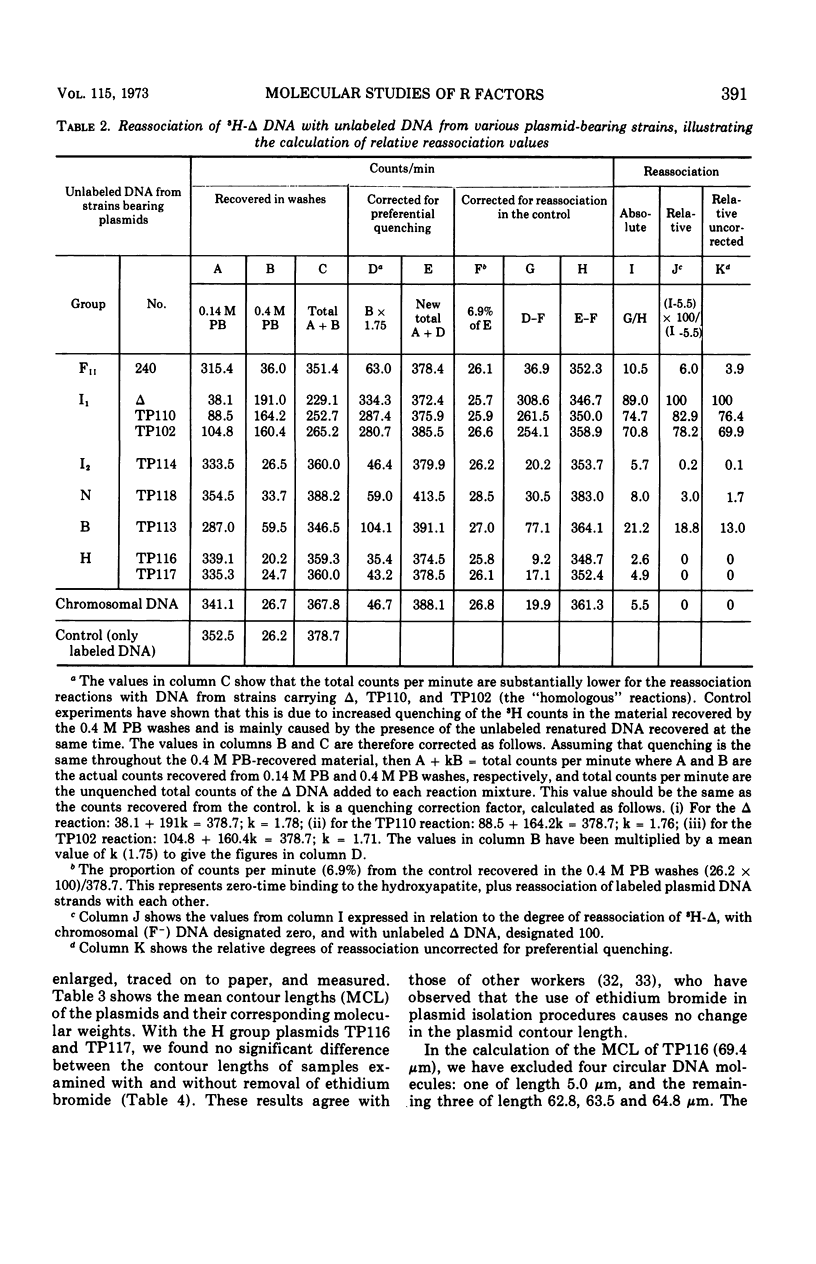
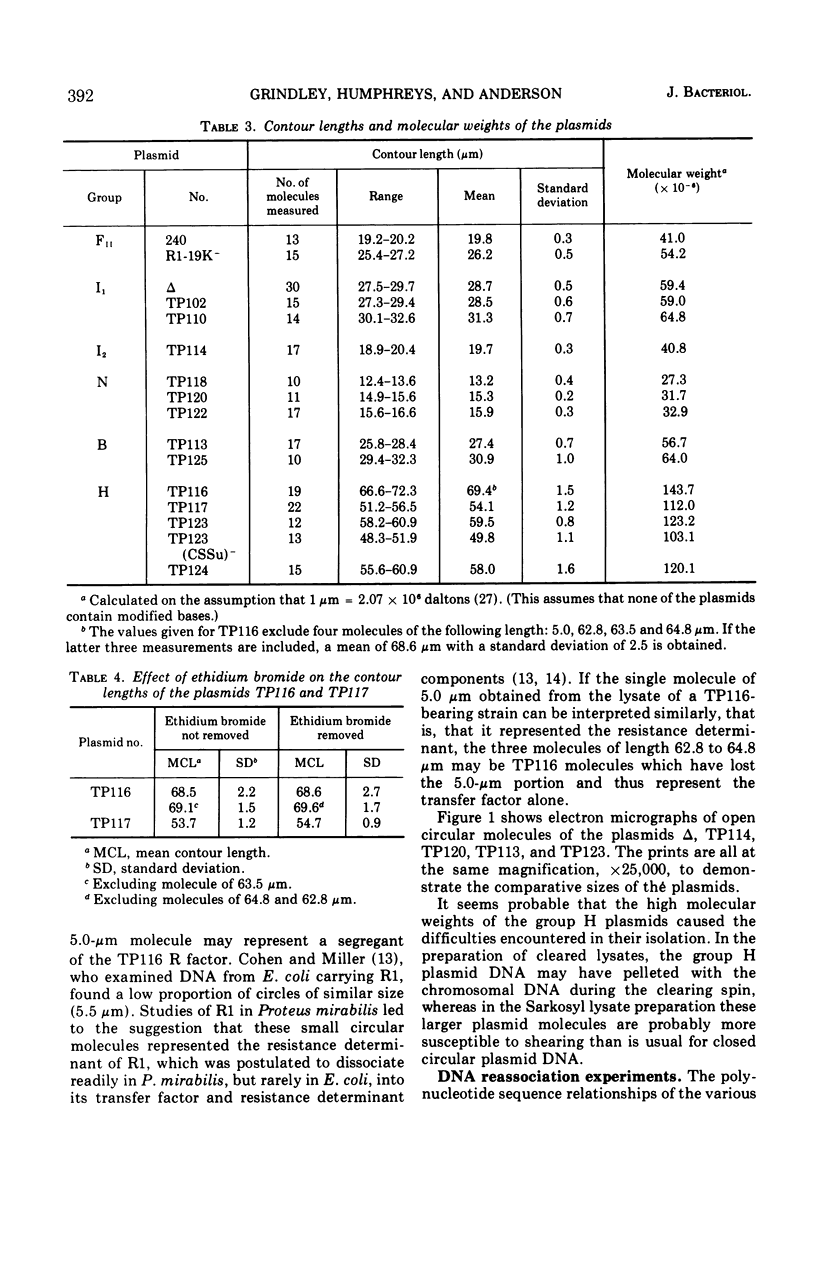
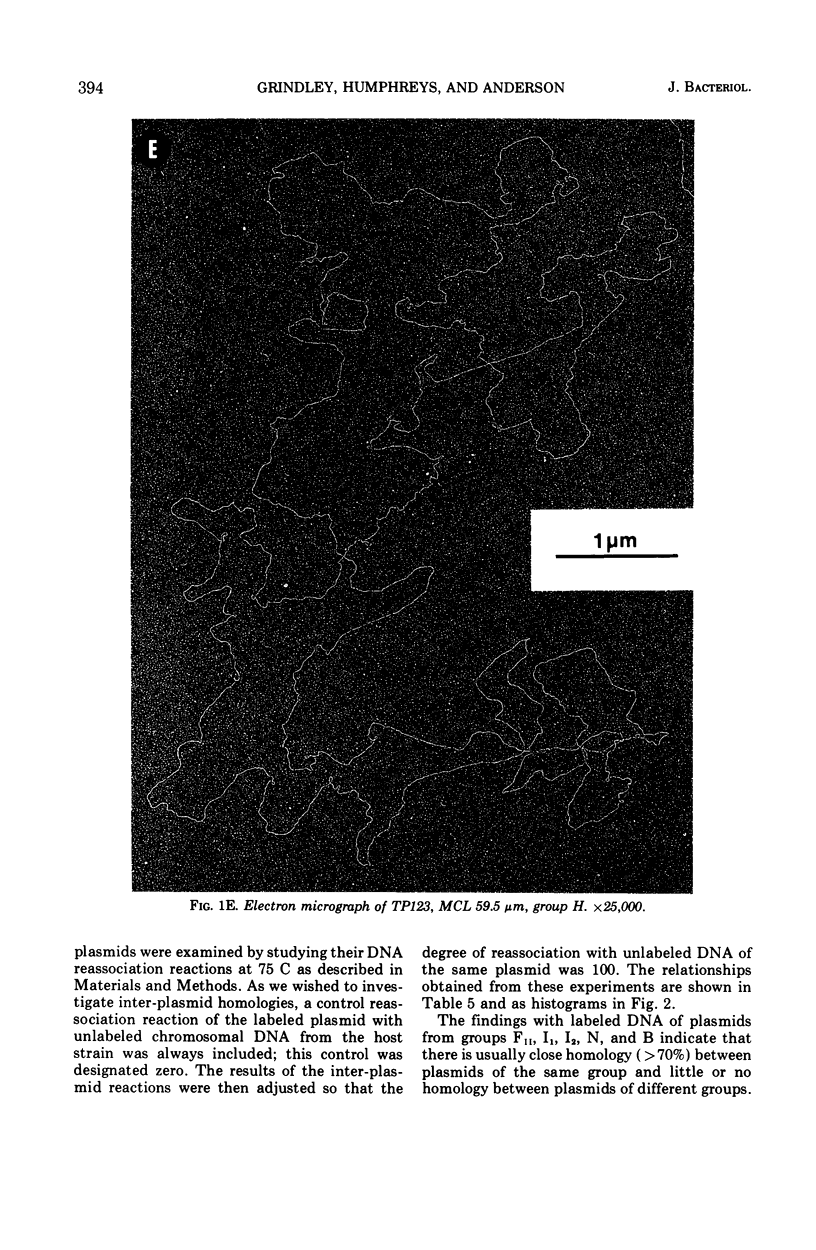
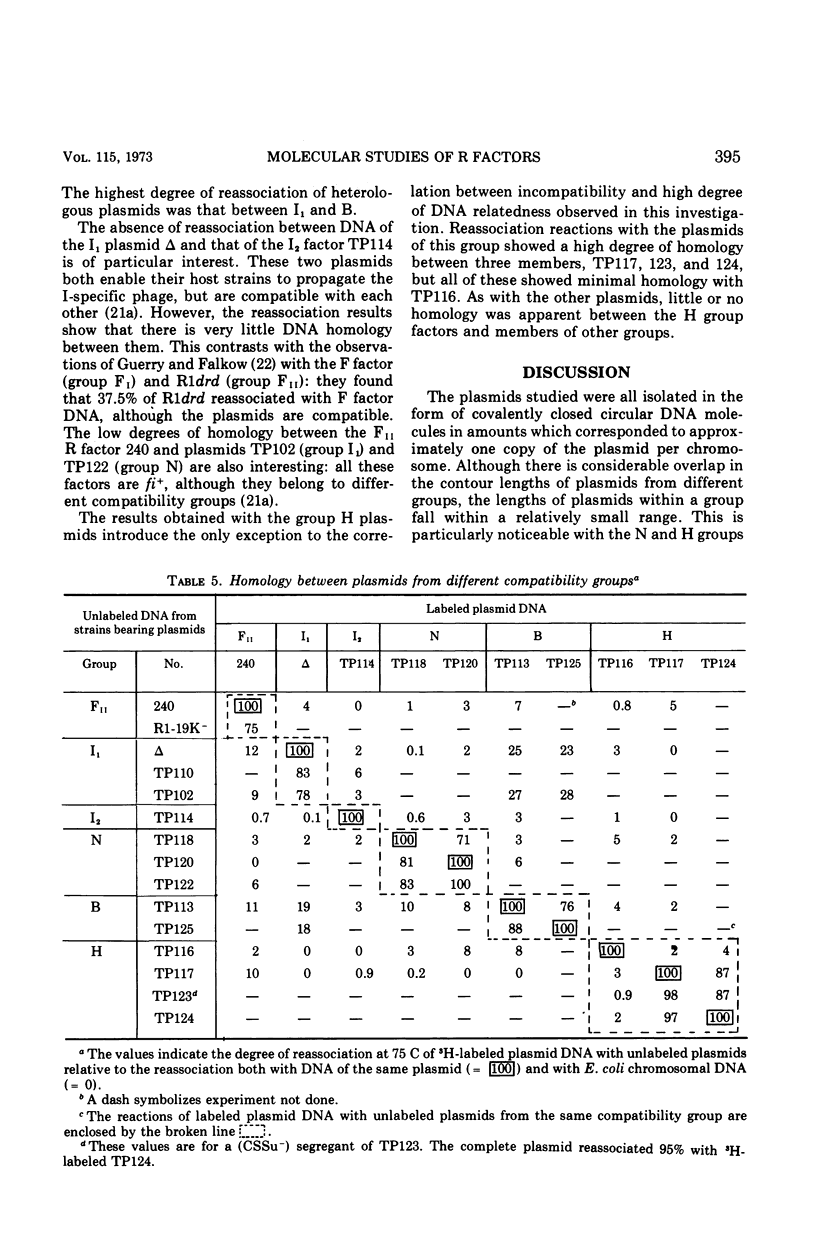
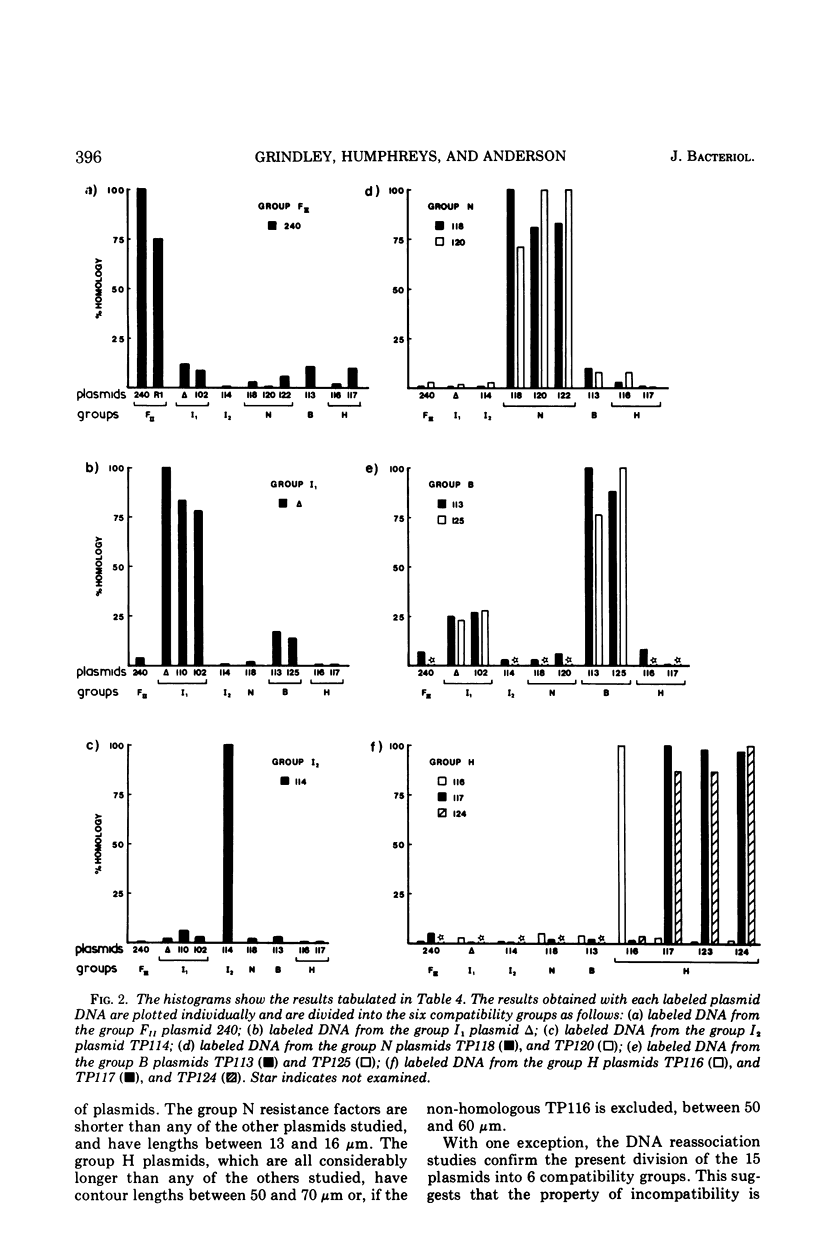
Molecular studies of R factors of six groups, FII, I1, I2, N, B, and H, defined on the basis of compatibility, support the conclusions drawn from genetic studies. In general, R factors of a given compatibility group are similar in size. Deoxyribonucleic acid (DNA) reassociation occurs freely between members of the same group but is minimal between heterologous groups. An exception to this was found in group H, of which one factor showed minimal homology with the remaining plasmids of the group. A further exception was found with groups I1 and B, which, although genetically distinct, show between 18 and 28% of DNA homology. Groups I1 and I2 are molecularly distinct, despite the fact that they both stimulate the synthesis of I-fimbriae.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S. Influence of the delta transfer factor on the phage sensitivity of salmonellae. Nature. 1966 Nov 19;212(5064):795–799. doi: 10.1038/212795a0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S., Lewis M. J. Characterization of a transfer factor associated with drug resistance in Salmonella typhimurium. Nature. 1965 Nov 27;208(5013):843–849. doi: 10.1038/208843a0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S., Smith H. R. Chloramphenicol resistance in the typhoid bacillus. Br Med J. 1972 Aug 5;3(5822):329–331. doi: 10.1136/bmj.3.5822.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. S., Threlfall E. J. Change of host range in a resistance factor. Genet Res. 1970 Oct 2;16(2):207–214. doi: 10.1017/s0016672300002421. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Bernardi G. Chromatography of nucleic acids on hydroxyapatite. Nature. 1965 May 22;206(4986):779–783. doi: 10.1038/206779a0. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Johnson K. E., Citarella R. V., Falkow S. Polynucleotide sequence relationships among members of Enterobacteriaceae. J Bacteriol. 1969 May;98(2):637–650. doi: 10.1128/jb.98.2.637-650.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Rake A. V., Johnson K. E. Batch procedure for thermal elution of DNA from hydroxyapatite. Anal Biochem. 1969 Apr 4;28(1):447–459. doi: 10.1016/0003-2697(69)90199-7. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Skerman F. J., Falkow S. Polynucleotide sequence divergence among strains of Escherichia coli and closely related organisms. J Bacteriol. 1972 Mar;109(3):953–965. doi: 10.1128/jb.109.3.953-965.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabbert Y. A., Scavizzi M. R., Witchitz J. L., Gerbaud G. R., Bouanchaud D. H. Incompatibility groups and the classification of fi - resistance factors. J Bacteriol. 1972 Nov;112(2):666–675. doi: 10.1128/jb.112.2.666-675.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee J. N., Datta N., Hedges R. W. R factors from Proteus rettgeri. J Gen Microbiol. 1972 Oct;72(3):543–552. doi: 10.1099/00221287-72-3-543. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Multiple molecular species of circular R-factor DNA isolated from Escherichia coli. Nature. 1969 Dec 27;224(5226):1273–1277. doi: 10.1038/2241273a0. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. II. Molecular nature of R-factors isolated from Proteus mirabilis and Escherichia coli. J Mol Biol. 1970 Jun 28;50(3):671–687. doi: 10.1016/0022-2836(70)90092-6. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Kelly R. B., Kornberg A. A minute circular DNA from Escherichia coli 15. Proc Natl Acad Sci U S A. 1968 Jul;60(3):992–999. doi: 10.1073/pnas.60.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Compatibility groups among fi - R factors. Nature. 1971 Nov 26;234(5326):222–223. doi: 10.1038/234222a0. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Kontomichalou P. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature. 1965 Oct 16;208(5007):239–241. doi: 10.1038/208239a0. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Electron microscopic study of the ethidium bromide-DNA complex. J Mol Biol. 1971 Sep 14;60(2):401–403. doi: 10.1016/0022-2836(71)90303-2. [DOI] [PubMed] [Google Scholar]
- Grindley J. N., Anderson E. S. I-like resistance factors with the fi+ character. Genet Res. 1971 Jun;17(3):267–271. doi: 10.1017/s0016672300012295. [DOI] [PubMed] [Google Scholar]
- Grindley N. D., Anderson E. S., Smith H. R., Grindley J. N. The effects of Salmonella typhimurium on derepressed mutants of F-like factors. Genet Res. 1971 Feb;17(1):89–93. doi: 10.1017/s0016672300012052. [DOI] [PubMed] [Google Scholar]
- Grindley N. D., Grindley J. N., Anderson E. S. R factor compatibility groups. Mol Gen Genet. 1972;119(4):287–297. doi: 10.1007/BF00272087. [DOI] [PubMed] [Google Scholar]
- Guerry P., Falkow S. Polynucleotide sequence relationships among some bacterial plasmids. J Bacteriol. 1971 Jul;107(1):372–374. doi: 10.1128/jb.107.1.372-374.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N. R124, an fi R factor of a new compatibility class. J Gen Microbiol. 1972 Jul;71(2):403–405. doi: 10.1099/00221287-71-2-403. [DOI] [PubMed] [Google Scholar]
- Khatoon H., Iyer R. V., Iyer V. N. A new filamentous bacteriophage with sex-factor specificity. Virology. 1972 Apr;48(1):145–155. doi: 10.1016/0042-6822(72)90122-5. [DOI] [PubMed] [Google Scholar]
- Khatoon H., Iyer R. V. Stable coexistence of Rfi factors in Escherichia coli. Can J Microbiol. 1971 May;17(5):669–675. doi: 10.1139/m71-108. [DOI] [PubMed] [Google Scholar]
- Lang D., Mitani M. Simplified quantitative electron microscopy of biopolymers. Biopolymers. 1970;9(3):373–379. doi: 10.1002/bip.1970.360090310. [DOI] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Meynell E., Cooke M. Repressor-minus and operator-constitutive de-repressed mutants of F-like R factors: their effect on chromosomal transfer by HfrC. Genet Res. 1969 Dec;14(3):309–313. doi: 10.1017/s0016672300002123. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. C. Molecular recombination between R-factor deoxyribonucleic acid molecules in Escherichia coli host cells. J Bacteriol. 1970 Jul;103(1):166–177. doi: 10.1128/jb.103.1.166-177.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchaudhuri S. R., Mazaitis A. J., Maas W. K., Kleinschmidt A. K. Characterization by electron microscopy of fused F-prime factors in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1873–1876. doi: 10.1073/pnas.69.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniker C. K., Vimala K. N. Transferable chloramphenicol resistance in Salmonella typhi. Nature. 1972 Sep 8;239(5367):109–110. doi: 10.1038/239109b0. [DOI] [PubMed] [Google Scholar]
- SCAIFE J., GROSS J. D. Inhibition of multiplication of an Flac factor in Hfr cells of Escherichia coli K-12. Biochem Biophys Res Commun. 1962 May 11;7:403–407. doi: 10.1016/0006-291x(62)90324-8. [DOI] [PubMed] [Google Scholar]
- WATANABE T., NISHIDA H., OGATA C., ARAI T., SATO S. EPISOME-MEDIATED TRANSFER OF DRUG RESISTANCE IN ENTEROBACTERIACEAE. VII. TWO TYPES OF NATURALLY OCCURRING R FACTORS. J Bacteriol. 1964 Sep;88:716–726. doi: 10.1128/jb.88.3.716-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]




