Abstract
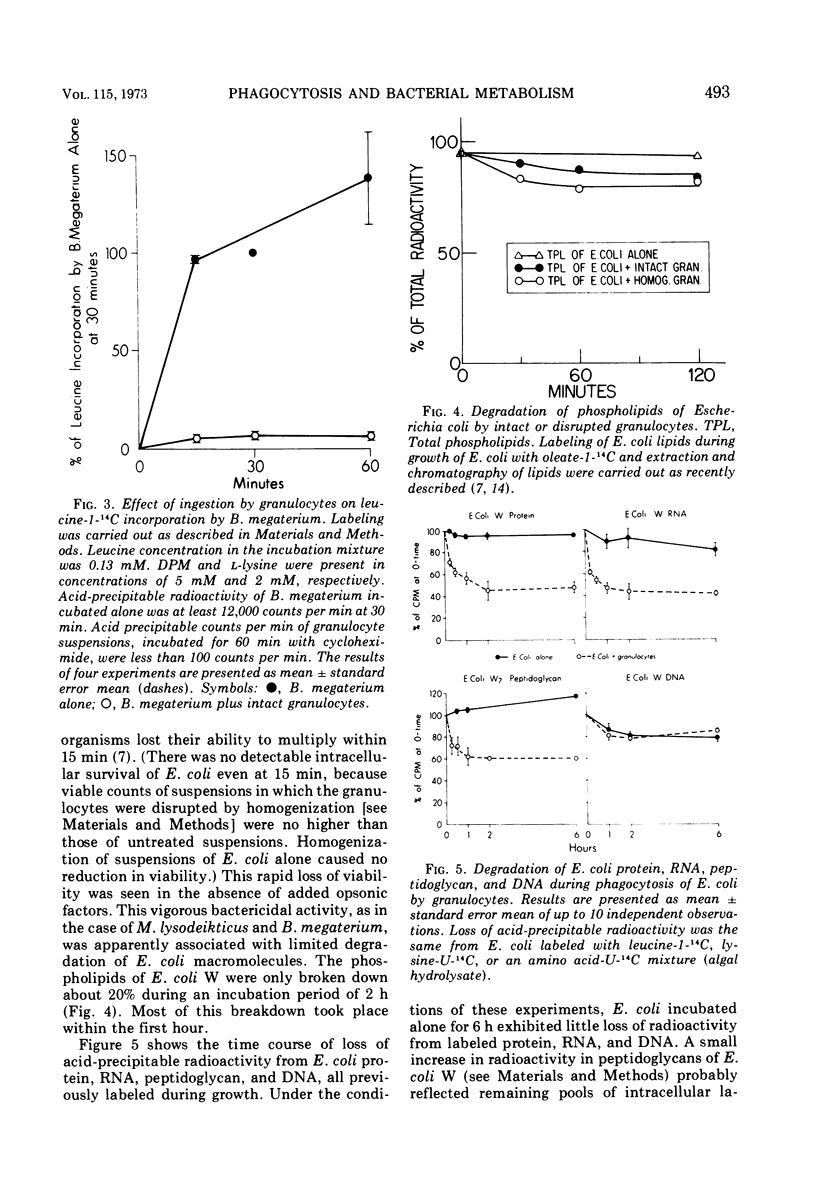
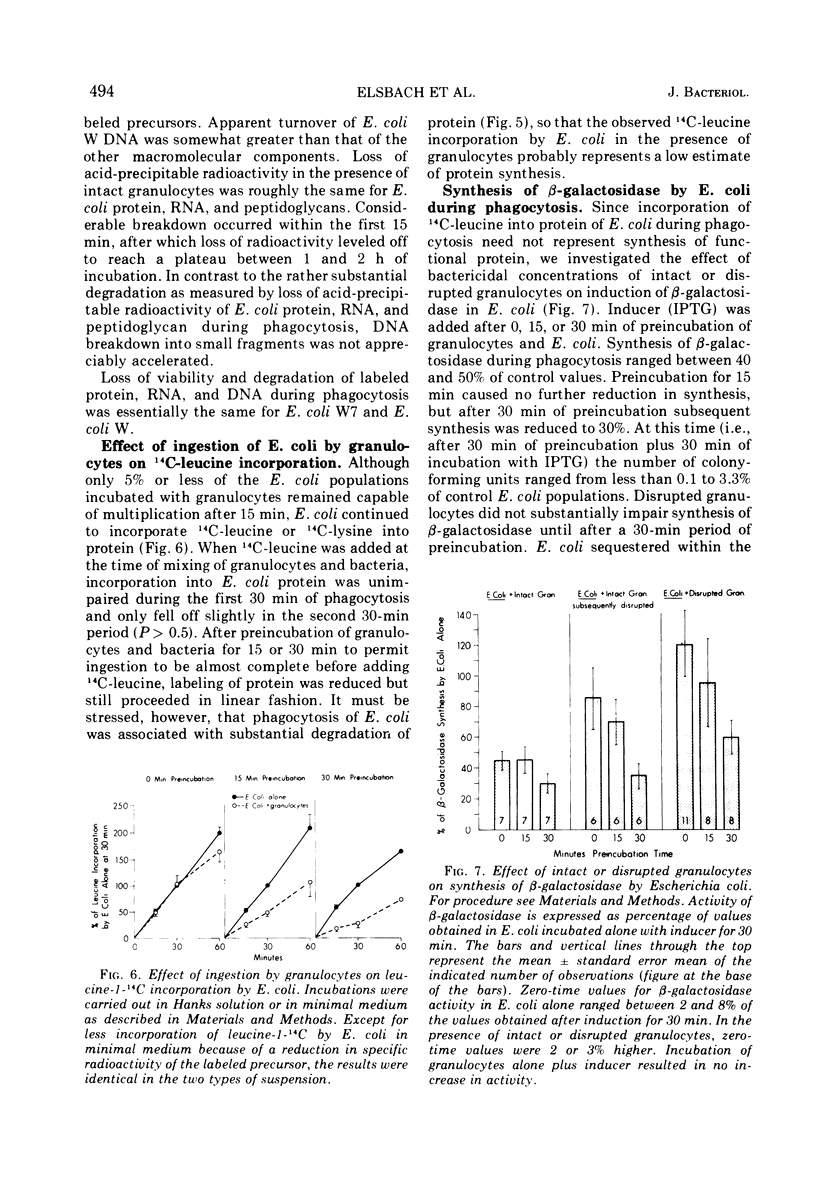
Phagocytosis and killing of gram-positive Bacillus megaterium and Micrococcus lysodeikticus by granulocytes in vitro is associated with almost immediate cessation of bacterial protein synthesis. By contrast, protein synthesis by Escherichia coli continues after ingestion and killing. After preincubation of E. coli with intact granulocytes for 15 min, when 95% or more of the bacteria can no longer multiply, induction of β-galactosidase proceeds at rates about half of control values. With disrupted granulocytes, which kill E. coli as rapidly as intact cells, the rate of induction of β-galactosidase does not fall until after 30 min of preincubation. We attribute the different effects of phagocytosis on the biochemical apparatus of these microorganisms to the different fates of their envelopes. Specifically labeled protein, ribonucleic acid, deoxyribonucleic acid, and lipid of all three species of bacteria and peptidoglycan of E. coli are apparently incompletely degraded during phagocytosis. However, the cell walls of M. lysodeikticus and B. megaterium undergo rapid and almost complete degradation. The resulting structural disintegration of these gram-positive microorganisms must cause extensive biochemical disorganization as well. Our evidence indicates that the E. coli envelope, on the other hand, retains sufficient structural organization to preserve integrated biochemical function for at least 1 h after the bacteria have lost the ability to multiply.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birdsell D. C., Cota-Robles E. H. Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol. 1967 Jan;93(1):427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budman D. R., Pardee A. B. Thymidine and thymine incorporation into deoxyribonucleic acid: inhibition and repression by uridine of thymidine phosphorylase of Escherichia coli. J Bacteriol. 1967 Nov;94(5):1546–1550. doi: 10.1128/jb.94.5.1546-1550.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A. The fate of bacteria within phagocytic cells. I. The degradation of isotopically labeled bacteria by polymorphonuclear leucocytes and macrophages. J Exp Med. 1963 Jan 1;117:27–42. doi: 10.1084/jem.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers D. A., Zubay G. The stimulatory effect of cyclic adenosine 3'5'-monophosphate on DNA-directed synthesis of beta-galactosidase in a cell-free system. Proc Natl Acad Sci U S A. 1969 May;63(1):118–122. doi: 10.1073/pnas.63.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Levy S. Increased synthesis of phospholipid during phagocytosis. J Clin Invest. 1968 Oct;47(10):2217–2229. doi: 10.1172/JCI105907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the action of serum and the role of lysozyme in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2118–2126. doi: 10.1128/jb.96.6.2118-2126.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the lethal event in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2127–2131. doi: 10.1128/jb.96.6.2127-2131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Intraleukocytic microbicidal defects. Annu Rev Med. 1971;22:39–62. doi: 10.1146/annurev.me.22.020171.000351. [DOI] [PubMed] [Google Scholar]
- Melching L., Vas S. I. Effects of serum components on gram-negative bacteria during bactericidal reactions. Infect Immun. 1971 Jan;3(1):107–115. doi: 10.1128/iai.3.1.107-115.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel D. W., Gilvarg C. Mucopeptide metabolism during growth and sporulation in Bacillus megaterium. J Biol Chem. 1970 Dec 25;245(24):6711–6717. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Scharff R., Hendler R. W., Nanninga N., Burgess A. H. Respiration and protein synthesis in Escherichia coli membrane-envelope fragments. IV. Chemical and cytological characterization and biosynthetic capabilities of fragments obtained by mild procedures. J Cell Biol. 1972 Apr;53(1):1–23. doi: 10.1083/jcb.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Perlman R. L., Pastan I. Regulation of lac transcription in Escherichia coli by cyclic adenosine 3',5'-monophosphate. Studies with deoxyribonucleic acid-ribonucleic acid hybridization and hybridization competition. J Biol Chem. 1970 Dec 10;245(23):6366–6372. [PubMed] [Google Scholar]
- Wurster N., Elsbach P., Rand J., Simon E. J. Effects of levorphanol on phospholipid metabolism and composition in Escherichia coli. Biochim Biophys Acta. 1971 Nov 5;248(2):282–292. doi: 10.1016/0005-2760(71)90016-6. [DOI] [PubMed] [Google Scholar]


