Abstract
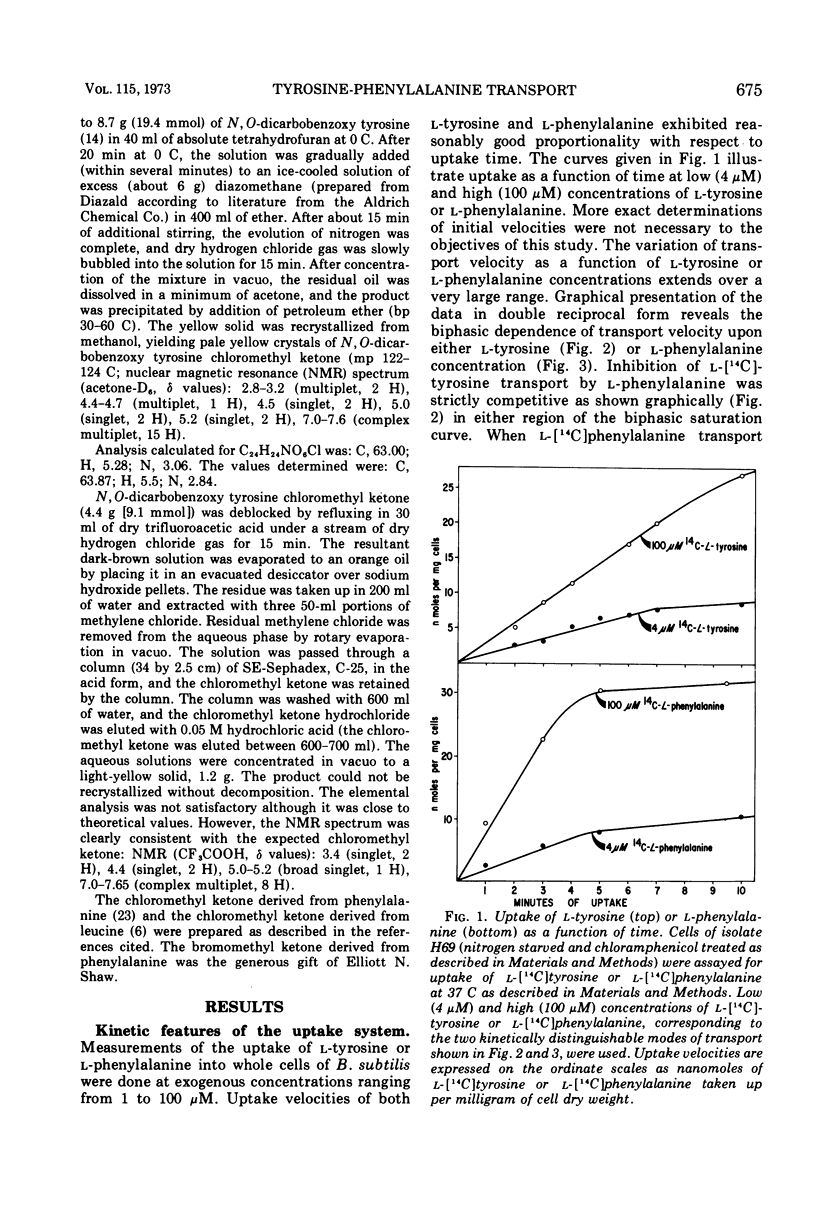
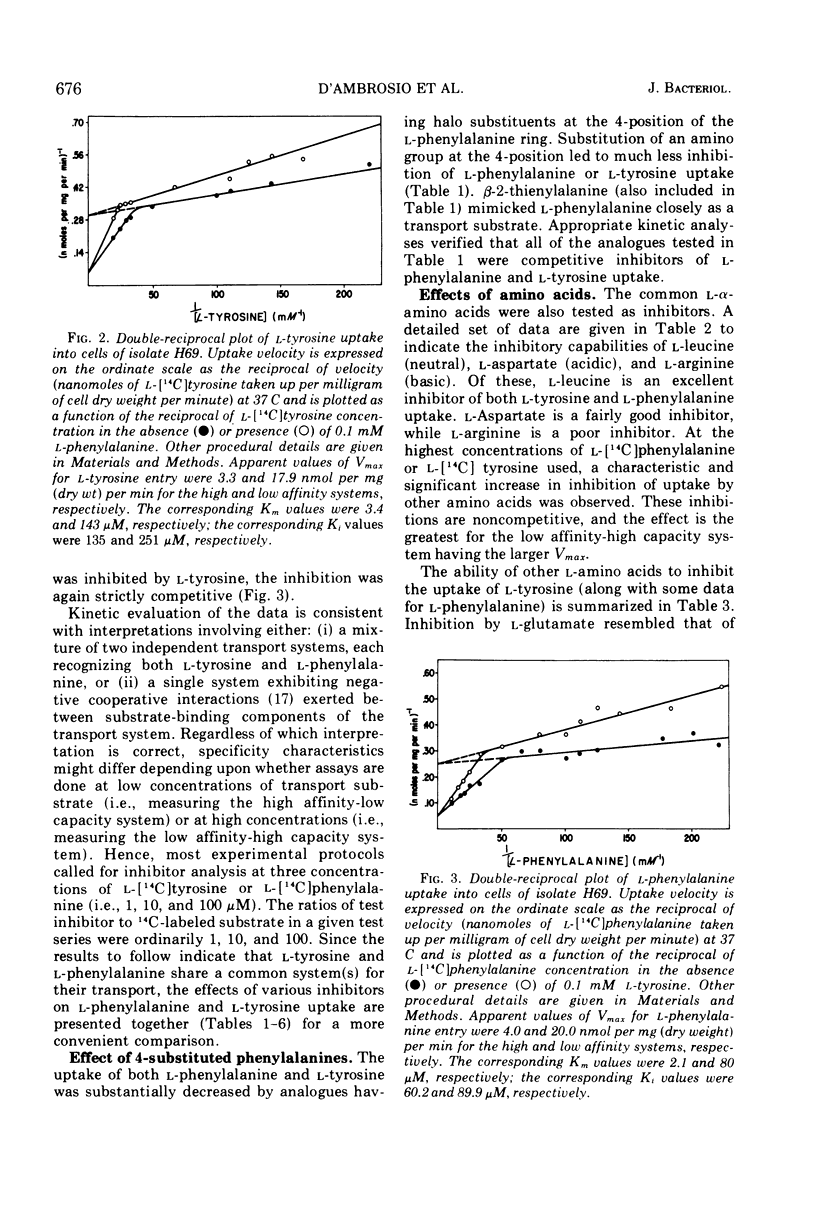
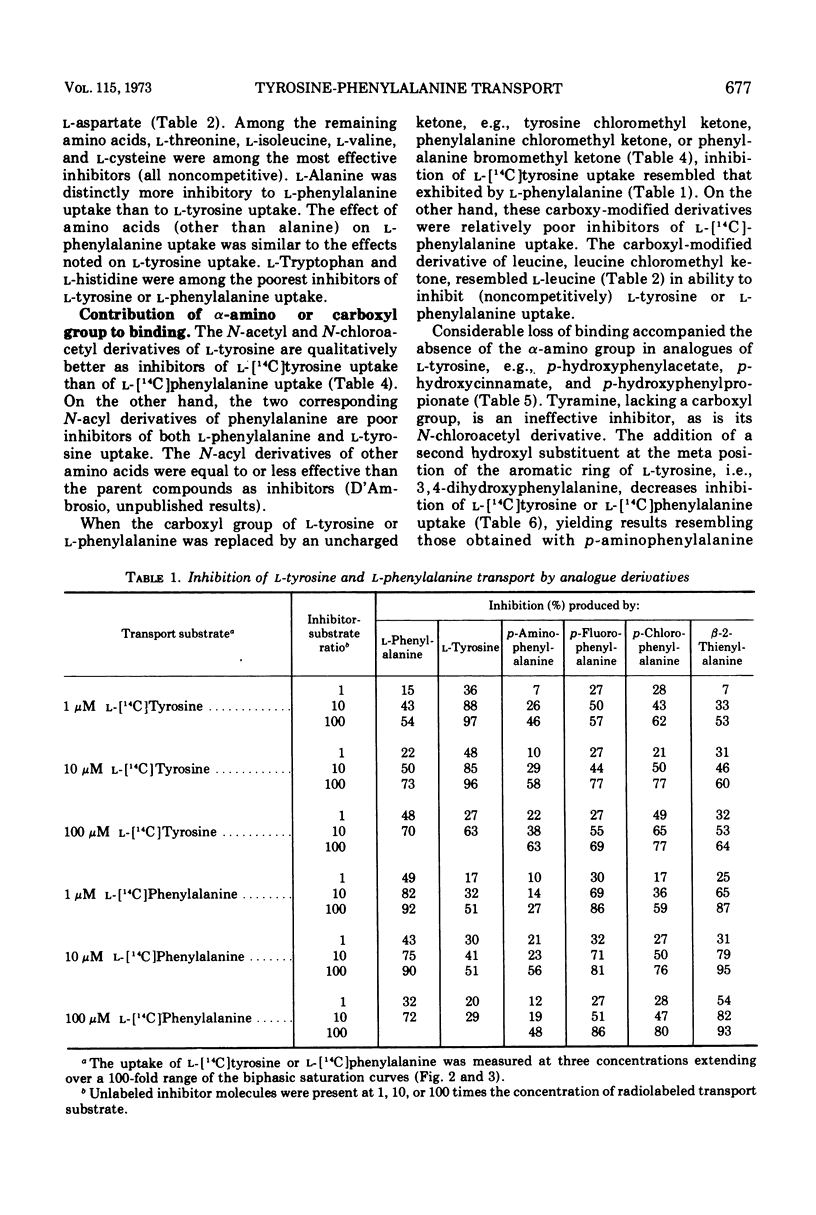
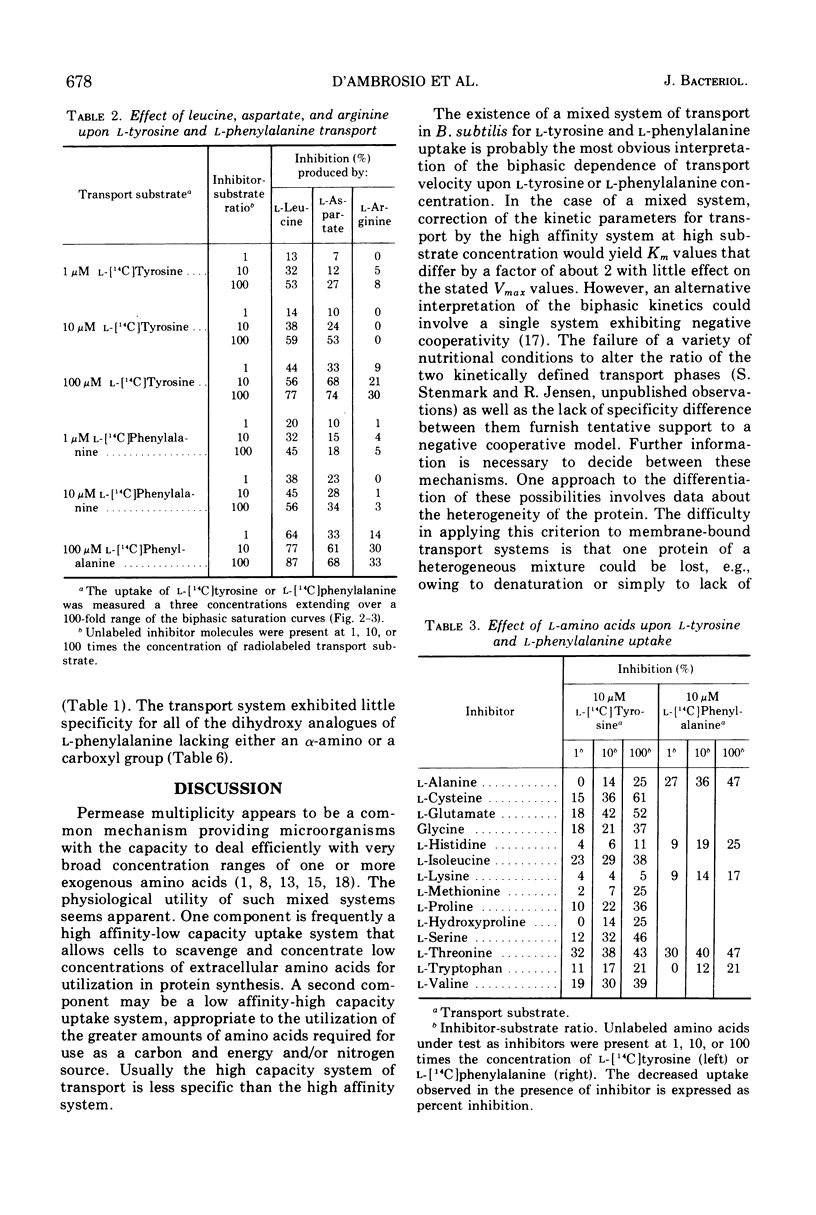
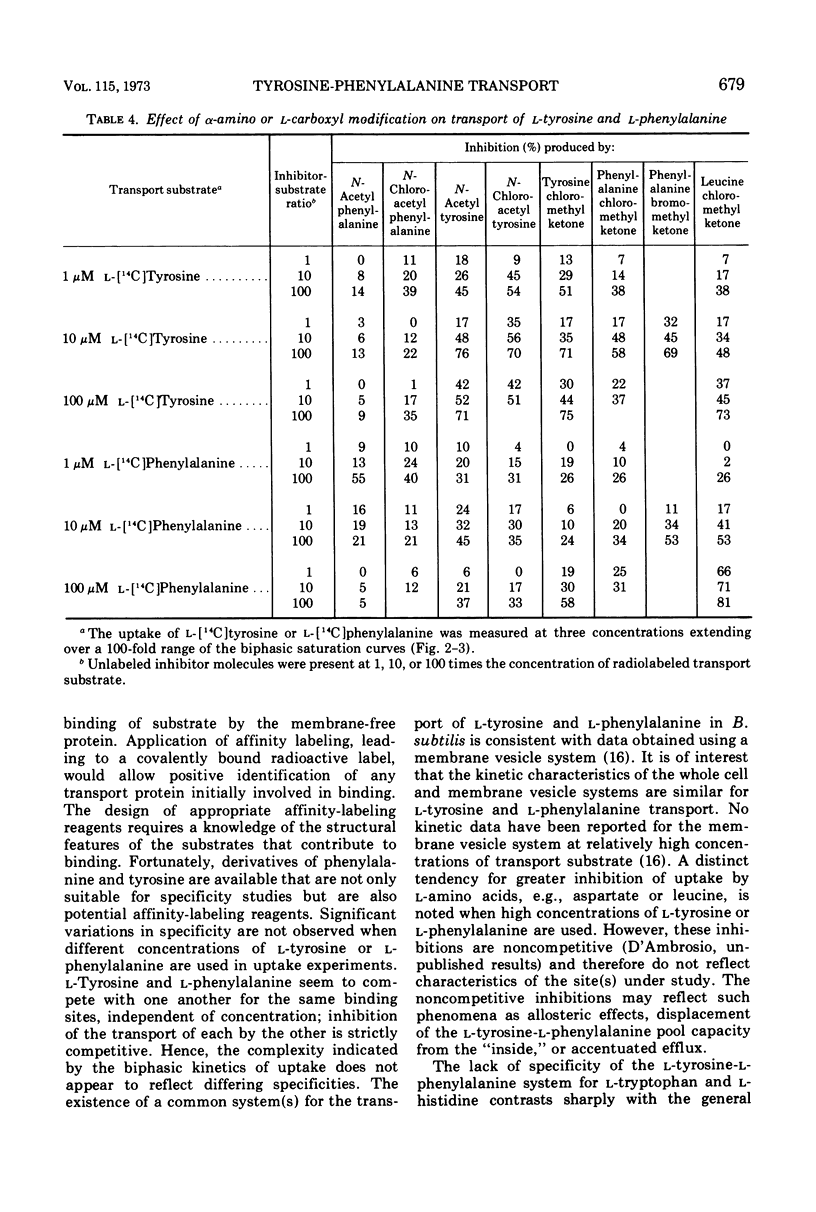
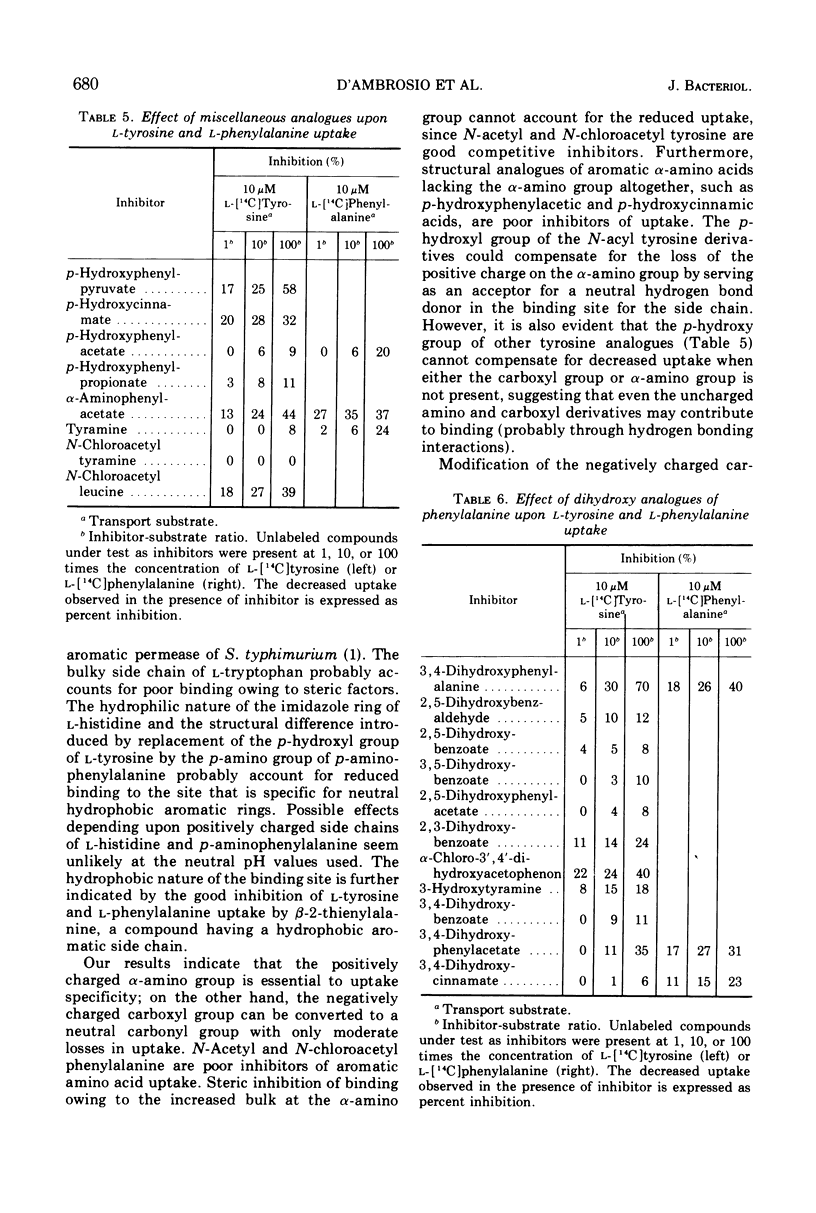
l-Tyrosine and l-phenylalanine enter cells of Bacillus subtilis via a system of active transport that exhibits complex kinetic behavior. The specificity of the transport system was characterized both at low concentrations of transport substrate (where affinity for l-tyrosine or l-phenylalanine is high but capacity is low) and at high concentrations (where affinity is low but capacity is high). Specificity was not found to differ significantly as a function of either l-tyrosine or l-phenylalanine concentration. Kinetic analysis showed that the relationship between the uptake of l-phenylalanine and l-tyrosine is strictly competitive. Neither l-tyrosine nor l-phenylalanine uptake was competitively inhibited by other naturally occurring l-amino acids, indicating the importance of the phenyl side chain to uptake specificity. Hence, it is concluded that l-tyrosine and l-phenylalanine are transported by a common system that is specific for these two amino acids. The abilities of analogue derivatives of l-tyrosine and l-phenylalanine to inhibit the uptake of l-[14C]tyrosine and l-[14C]phenylalanine competitively were determined throughout a wide range of substrate and inhibitor concentrations. In this manner, the contributions of the side chain, the α-amino group and the carboxyl group to uptake specificity were established. It is concluded that the positively charged α-amino group contributes more significantly to uptake specificity than does the negatively charged carboxyl group. The recognition of a phenyl ring is an essential feature of specificity; other amino acids with aromatic side chains, such as the indole and imidazole rings of l-tryptophan and l-histidine, do not compete with l-tyrosine and l-phenylalanine for uptake. The presence of the p-hydroxy substitutent in the side chain (as in l-tyrosine) enhances the uptake of the aryl amino acid analogues investigated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Roth J. R. Histidine and aromatic permeases of Salmonella typhimurim. J Bacteriol. 1968 Nov;96(5):1742–1749. doi: 10.1128/jb.96.5.1742-1749.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J. M., Wilchek M., Katchalski E. Irreversible inhibition of biotin transport in yeast by biotinyl-p-nitrophenyl ester. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2604–2607. doi: 10.1073/pnas.68.10.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin R. D., Stadtman E. R. A possible role of purine nucleotide pyrophosphorylases in the regulation of purine uptake by Bacillus subtilis. J Biol Chem. 1966 Jun 10;241(11):2679–2686. [PubMed] [Google Scholar]
- Bernlohr R. W. Changes in amino acid permeation during sporulation. J Bacteriol. 1967 Mar;93(3):1031–1044. doi: 10.1128/jb.93.3.1031-1044.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch P. L., el-Obeid H. A., Akhtar M. The preparation of chloromethylketone analogues of amino acids: inhibition of leucine aminopeptidase. Arch Biochem Biophys. 1972 Feb;148(2):447–451. doi: 10.1016/0003-9861(72)90163-4. [DOI] [PubMed] [Google Scholar]
- Champney W. S., Jensen R. A. D-Tyrosine as a metabolic inhibitor of Bacillus subtilis. J Bacteriol. 1969 Apr;98(1):205–214. doi: 10.1128/jb.98.1.205-214.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Carter J. R., Kennedy E. P. GENETIC CONTROL OF THE MEMBRANE PROTEIN COMPONENT OF THE LACTOSE TRANSPORT SYSTEM OF Escherichia coli. Proc Natl Acad Sci U S A. 1967 Mar;57(3):698–705. doi: 10.1073/pnas.57.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Hou C., Crabeel M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. IV. Evidence for a general amino acid permease. J Bacteriol. 1970 Sep;103(3):770–777. doi: 10.1128/jb.103.3.770-777.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A. A biochemical basis for apparent abortive transformation in Bacillus subtilis. Genetics. 1968 Dec;60(4):707–717. doi: 10.1093/genetics/60.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Stenmark S. L., Champney W. S. Molecular basis for the differential anti-metabolite action of D-tyrosine in strains 23 and 168 of Bacillus subtilis. Arch Mikrobiol. 1972;87(2):173–180. doi: 10.1007/BF00424998. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport. Annu Rev Biochem. 1970;39:561–598. doi: 10.1146/annurev.bi.39.070170.003021. [DOI] [PubMed] [Google Scholar]
- Kay W. W. Two aspartate transport systems in Escherichia coli. J Biol Chem. 1971 Dec 10;246(23):7373–7382. [PubMed] [Google Scholar]
- Konings W. N., Freese E. Amino acid transport in membrane vesicles of Bacillus subtilis. J Biol Chem. 1972 Apr 25;247(8):2408–2418. [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr Negative cooperativity in regulatory enzymes. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1121–1128. doi: 10.1073/pnas.62.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. C. The genetics of bacterial transport systems. Annu Rev Genet. 1970;4:225–262. doi: 10.1146/annurev.ge.04.120170.001301. [DOI] [PubMed] [Google Scholar]
- Magill C. W., Sweeney H., Woodward V. W. Histidine uptake in strains of Neurospora crassa with normal and mutant transport systems. J Bacteriol. 1972 Apr;110(1):313–320. doi: 10.1128/jb.110.1.313-320.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino acid transport systems in Escherichia coli K-12. J Biol Chem. 1968 Nov 25;243(22):5914–5920. [PubMed] [Google Scholar]
- Shaw E., Ruscica J. The reactivity of His-57 in chymotrypsin to alkylation. Arch Biochem Biophys. 1971 Aug;145(2):484–489. doi: 10.1016/s0003-9861(71)80008-5. [DOI] [PubMed] [Google Scholar]
- Shaw E. Selective chemical modification of proteins. Physiol Rev. 1970 Apr;50(2):244–296. doi: 10.1152/physrev.1970.50.2.244. [DOI] [PubMed] [Google Scholar]
- Willecke K., Pardee A. B. Inducible transport of citrate in a Gram-positive bacterium, Bacillus subtilis. J Biol Chem. 1971 Feb 25;246(4):1032–1040. [PubMed] [Google Scholar]


