Abstract
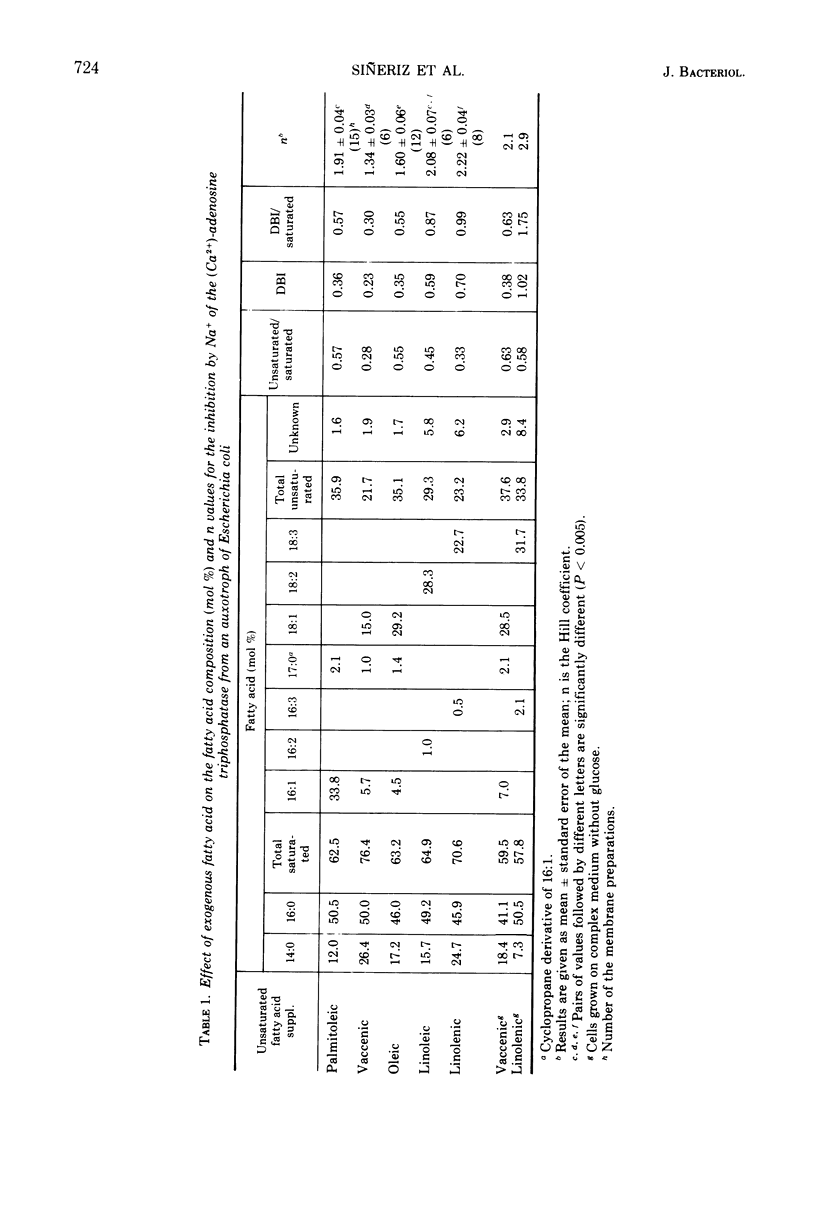
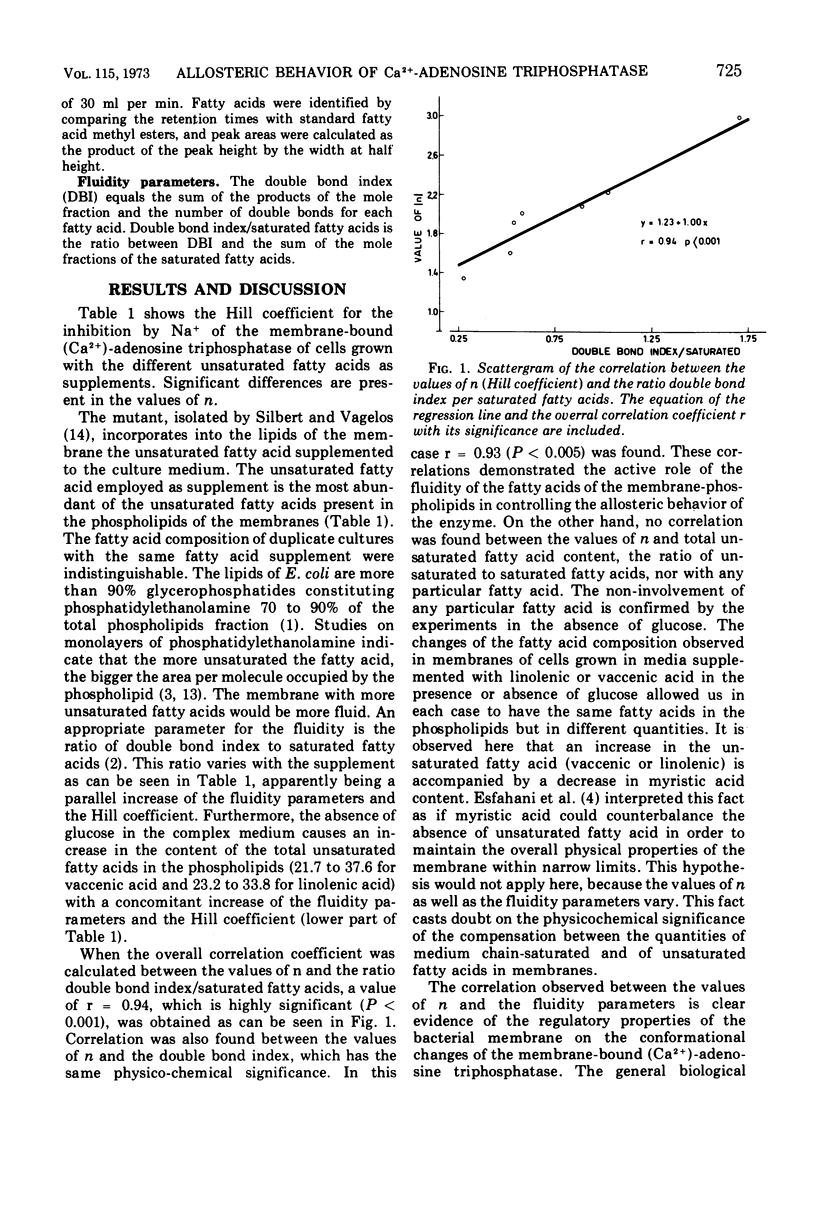
The allosteric properties of the membrane-bound (Ca2+)-adenosine triphosphatase of an unsaturated fatty acid auxotroph of Escherichia coli were studied in membranes with different fatty acid compositions. The Hill coefficient of the inhibition by Na+ ranged from 1.4, in the case where the auxotroph was grown with cis-vaccenic acid as supplement, to 2.8 when grown on linolenic acid. The results indicate that no fatty acid is particularly involved in the allosteric phenomena. A correlation between the values of the Hill coefficient and the double bond index or the ratio of the double bond index saturated to the fatty acids of the membrane was found. These facts are interpreted as a modulation by the membrane fluidity of the allosteric behavior of the membrane-bound enzyme. The general biological character of this phenomenon is discussed in this paper.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloj B., Morero R. D., Farías R. N., Trucco R. E. Membrane lipid fatty acids and regulation of membrane-bound enzymes. Allosteric behaviour of erythrocyte Mg 2+ -ATPase, (Na + +K + )-ATPase and acetylcholinesterase from rats fed different fat-supplemented diets. Biochim Biophys Acta. 1973 Jun 7;311(1):67–79. doi: 10.1016/0005-2736(73)90255-1. [DOI] [PubMed] [Google Scholar]
- Esfahani M., Barnes E. M., Jr, Wakil S. J. Control of fatty acid composition in phospholipids of Escherichia coli: response to fatty acid supplements in a fatty acid auxotroph. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1057–1064. doi: 10.1073/pnas.64.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr Membrane adenosine triphosphatase of Escherichia coli: activation by calcium ion and inhibition by monovalent cations. J Bacteriol. 1969 Nov;100(2):914–922. doi: 10.1128/jb.100.2.914-922.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Farias R. N., Goldemberg A. L., Trucco R. E. The effect of fat deprivation on the allosteric inhibition by fluoride of the (Mg2+)-ATPase and (Na+ and K+)-ATPase from rat erythrocytes. Arch Biochem Biophys. 1970 Jul;139(1):38–44. doi: 10.1016/0003-9861(70)90042-1. [DOI] [PubMed] [Google Scholar]
- Farías R. N., Londero L., Trucco R. E. Effect of membrane lipid composition on the allosteric inhibition by sodium of the Ca 2+ )-adenosine triphosphatase from Escherichia coli. J Bacteriol. 1972 Jan;109(1):471–473. doi: 10.1128/jb.109.1.471-473.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldemberg A. L., Farias R. N., Trucco R. E. Alosteric transitions and membrane-bound ATPase from rat tissues: the effect of fat deprivation on the allosteric inhibition by fluoride. Biochim Biophys Acta. 1973 Jan 26;291(2):489–493. doi: 10.1016/0005-2736(73)90500-2. [DOI] [PubMed] [Google Scholar]
- Goldemberg A. L., Farías R. N., Trucco R. E. Allosteric changes of p-nitrophenylphosphatase from rat erythrocytes in fat deficiency. J Biol Chem. 1972 Jul 10;247(13):4299–4304. [PubMed] [Google Scholar]
- Morero R. D., Bloj B., Farías R. N., Trucco R. E. The allosteric transitions from membrane-bound enzymes: behavior of erythrocyte acetylcholinesterase from fat-deficient rats. Biochim Biophys Acta. 1972 Sep 1;282(1):157–165. doi: 10.1016/0005-2736(72)90319-7. [DOI] [PubMed] [Google Scholar]
- Overath P., Schairer H. U., Stoffel W. Correlation of in vivo and in vitro phase transitions of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1970 Oct;67(2):606–612. doi: 10.1073/pnas.67.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert D. F., Vagelos P. R. Fatty acid mutant of E. coli lacking a beta-hydroxydecanoyl thioester dehydrase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1579–1586. doi: 10.1073/pnas.58.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]


