Abstract
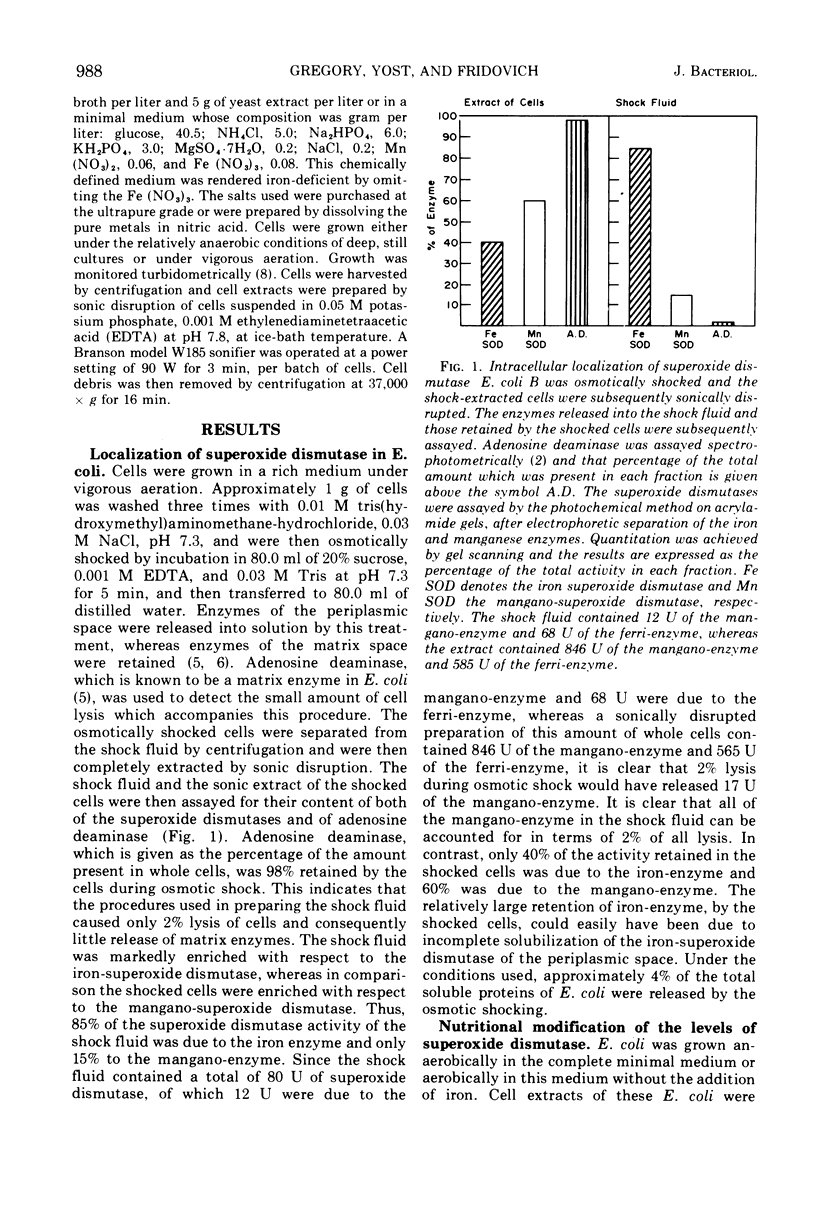
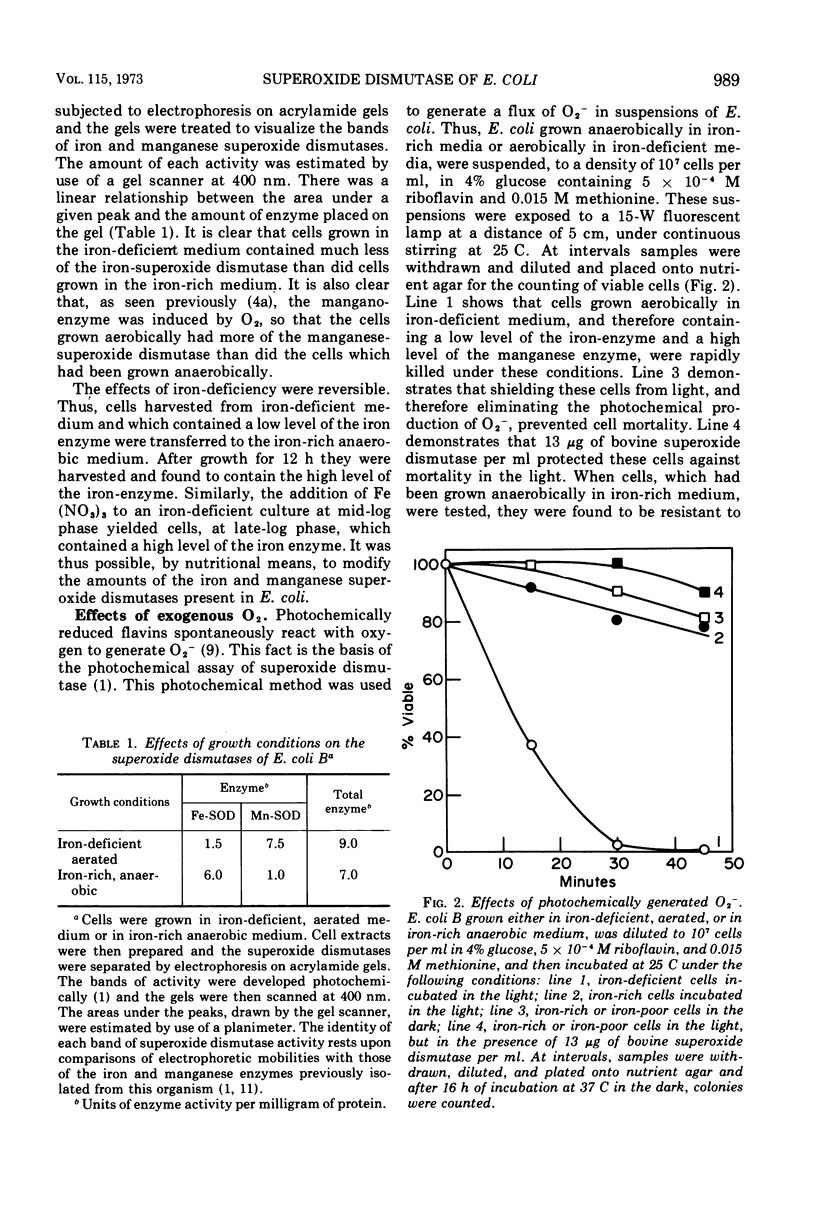
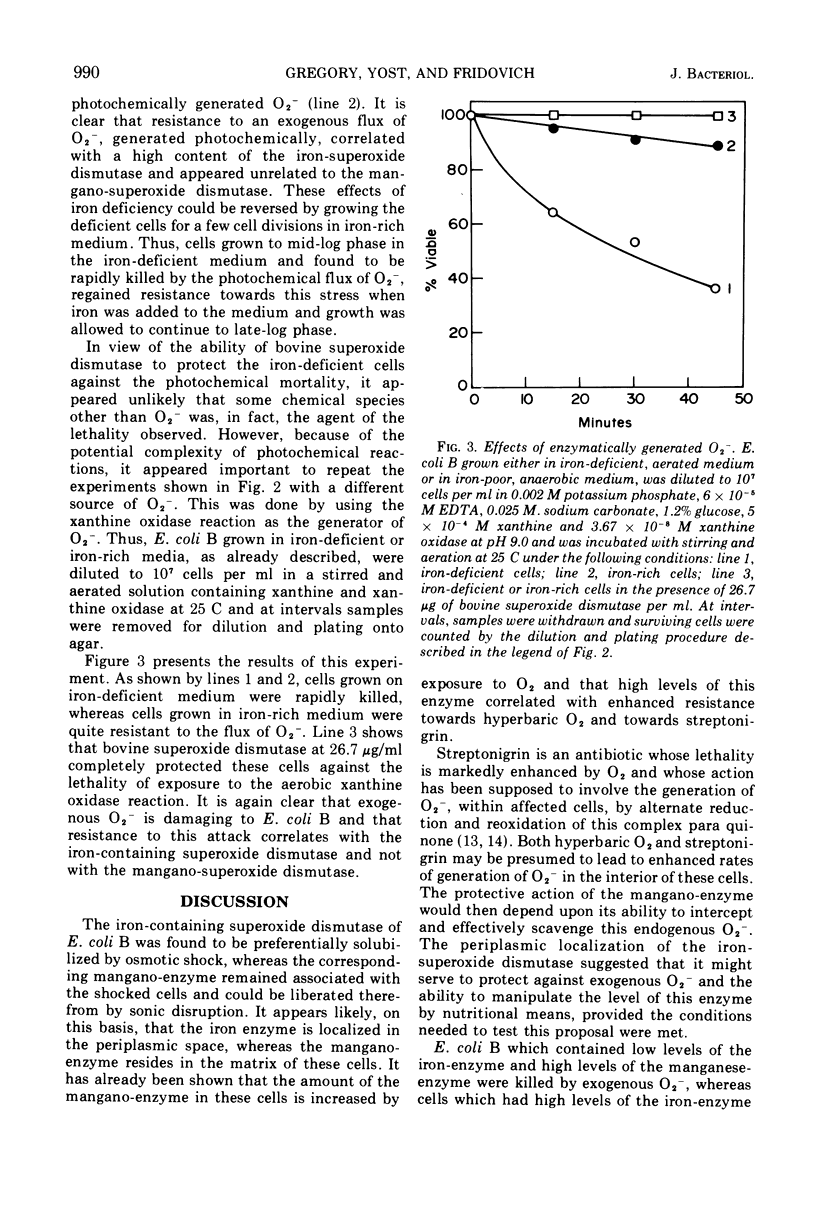
Escherichia coli B contains two superoxide dismutases which differ with respect to their localization within the cell, the nature of their prosthetic metals, their responses to changes in pO2, and their functions. One of these enzymes, which was liberated from the cells by osmotic shock and which was therefore presumed to be localized in the periplasmic space, is an iron-containing superoxide dismutase. The amount of this iron enzyme did not vary in response to changes in pO2 during growth. In contrast, the other superoxide dismutase was not solubilized by osmotic shock, was a mangano-protein, and was found in greater amounts in cells which had been grown at high pO2. E. coli, which had low levels of the iron-enzyme and high levels of the mangano-enzyme, as a consequence of growth in iron-deficient aerated medium, was killed by exposure to an exogenous flux of O2− which was generated either photochemically or enzymatically. The addition of bovine superoxide dismutase to the suspending medium protected these cells against this stress. On the other hand, E. coli, which had high levels of the iron-enzyme and low levels of the mangano-enzyme, as a consequence of growth in iron-rich anaerobic medium, was resistant to exogeneous O2−. On the basis of these and of previously reported results (4a, Yost, F. J. and I. Fridovich, J. Biol. Chem., 1973, in press), it appears that the iron superoxide dismutase, of the periplasmic space, serves as a defense against exogenous O2−, whereas the mangano-superoxide dismutase, in the matrix of these cells, serves to counter the toxicity of endogenous O2−.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen toxicity and the superoxide dismutase. J Bacteriol. 1973 Jun;114(3):1193–1197. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970 Nov 25;245(22):6176–6181. [PubMed] [Google Scholar]
- Koch A. L. Turbidity measurements of bacterial cultures in some available commercial instruments. Anal Biochem. 1970 Nov;38(1):252–259. doi: 10.1016/0003-2697(70)90174-0. [DOI] [PubMed] [Google Scholar]
- Massey V., Strickland S., Mayhew S. G., Howell L. G., Engel P. C., Matthews R. G., Schuman M., Sullivan P. A. The production of superoxide anion radicals in the reaction of reduced flavins and flavoproteins with molecular oxygen. Biochem Biophys Res Commun. 1969 Sep 10;36(6):891–897. doi: 10.1016/0006-291x(69)90287-3. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. L., White J. R. Interaction of streptonigrin with DNA in vitro. Biochim Biophys Acta. 1966 Sep;123(3):648–651. doi: 10.1016/0005-2787(66)90241-3. [DOI] [PubMed] [Google Scholar]
- White J. R., Dearman H. H. Generation of free radicals from phenazine methosulfate, streptonigrin, and riboflavin in bacterial suspensions. Proc Natl Acad Sci U S A. 1965 Sep;54(3):887–891. doi: 10.1073/pnas.54.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]


