Abstract
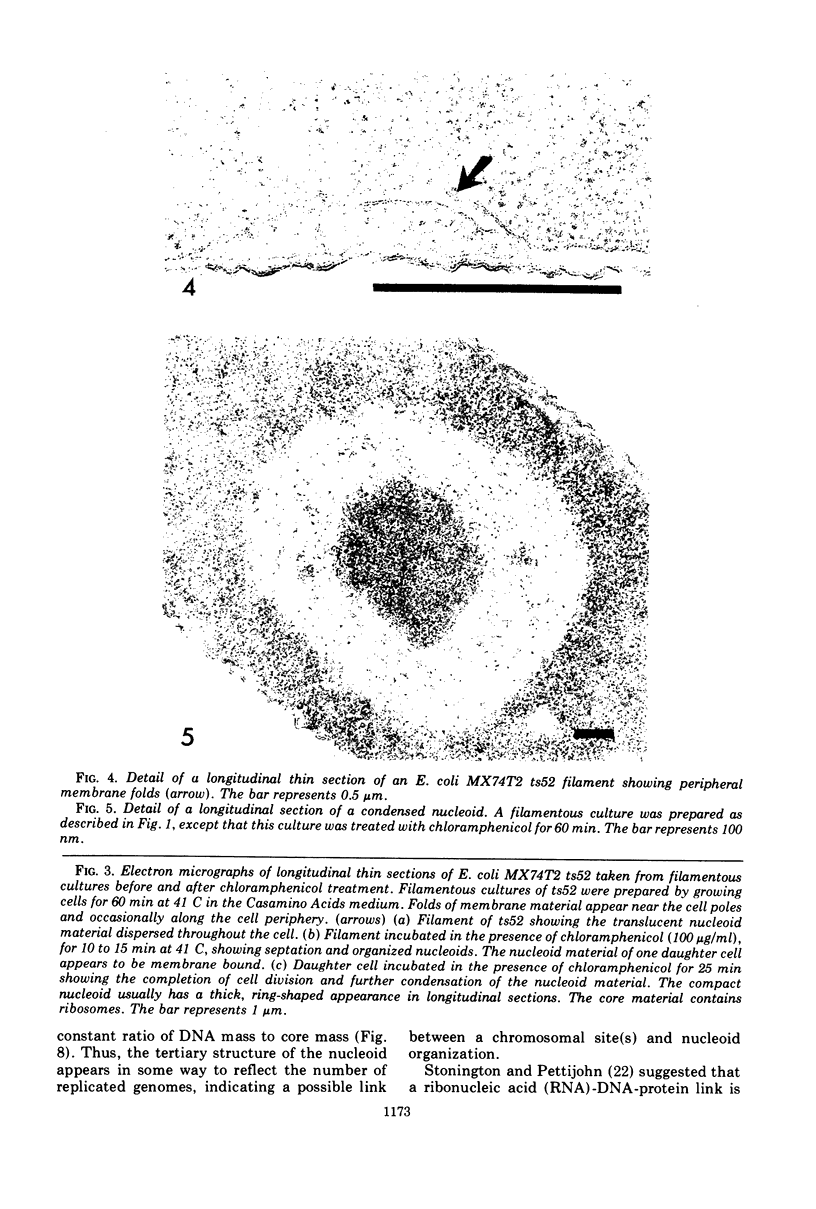
The reorganization of the bacterial nucleoid of an Escherichia coli mutant, MX74T2 ts52, was studied by electron microscopy after protein synthesis inhibition by using whole mounts of cell ghosts, ultrathin-sectioning, and freeze-etching. The bacterial nucleoid showed two morphological changes after chloramphenicol addition: deoxyribonucleic acid (DNA) localization and DNA condensation. DNA localization was observed 10 min after chloramphenicol addition; the DNA appeared as a compact, solid mass. DNA condensation was observed at 25 min; the nucleoid appeared as a cytoplasm-filled sphere, often opened at one end. Ribosomes were observed in the center. Giant nucleoids present in some mutant filaments showed fused, spherical nucleoids arranged linearly, suggesting that the tertiary structure of the nucleoid reflects the number of replicated genomes. Inhibitors which directly or indirectly blocked protein synthesis and caused DNA condensation were chloramphenicol, puromycin, amino acid starvation, rifampicin, or carbonyl cyanide m-chlorophenyl hydrazone. All inhibitors that caused cell division in the mutant also caused condensation, although some inhibitors caused condensation without cell division. Nucleoid condensation appears to be related to chromosome structure rather than to DNA segregation upon cell division.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bremer H., Yuan D. RNA chain growth-rate in Escherichia coli. J Mol Biol. 1968 Dec 14;38(2):163–180. doi: 10.1016/0022-2836(68)90404-x. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- Daneo-Moore L., Higgins M. L. Morphokinetic reaction of Streptococcus faecalis (ATCC 9790) cells to the specific inhibition of macromolecular synthesis: nucleoid condensation on the inhibition of protein synthesis. J Bacteriol. 1972 Mar;109(3):1210–1220. doi: 10.1128/jb.109.3.1210-1220.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison J. S., Mattern C. F., Daniel W. A. Structural changes in Clostridium botulinum type E after treatment with boticin S5 1 . J Bacteriol. 1971 Oct;108(1):526–534. doi: 10.1128/jb.108.1.526-534.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhs G. W. Symposium on the fine structure and replication of bacteria and their parts. I. Fine structure and replication of bacterial nucleoids. Bacteriol Rev. 1965 Sep;29(3):277–293. doi: 10.1128/br.29.3.277-293.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Inouye M., Pardee A. B. Changes of membrane proteins and their relation to deoxyribonucleic acid synthesis and cell division of Escherichia coli. J Biol Chem. 1970 Nov 10;245(21):5813–5819. [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A. Contribution à l'étude du noyau bactérien. Schweiz Z Pathol Bakteriol. 1955;18(5):1122–1137. [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEPES A. KINETICS OF INDUCED ENZYME SYNTHESIS. DETERMINATION OF THE MEAN LIFE OF GALACTOSIDASE-SPECIFIC MESSENGER RNA. Biochim Biophys Acta. 1963 Oct 15;76:293–309. [PubMed] [Google Scholar]
- MASON D. J., POWELSON D. M. Nuclear division as observed in live bacteria by a new technique. J Bacteriol. 1956 Apr;71(4):474–479. doi: 10.1128/jb.71.4.474-479.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rosenkranz H. S., Carr H. S., Rose H. M. Electron microscopy of chloramphenicol-treated Escherichia coli. J Bacteriol. 1967 Jun;93(6):1987–2002. doi: 10.1128/jb.93.6.1987-2002.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rosenkranz H. S. Ultrastructure of Escherichia coli depleted of an amino acid. J Bacteriol. 1970 May;102(2):584–587. doi: 10.1128/jb.102.2.584-587.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINOW C. F. The chromatin bodies of bacteria. Bacteriol Rev. 1956 Dec;20(4):207–242. doi: 10.1128/br.20.4.207-242.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SADLER J. R., NOVICK A. THE PROPERTIES OF REPRESSOR AND THE KINETICS OF ITS ACTION. J Mol Biol. 1965 Jun;12:305–327. doi: 10.1016/s0022-2836(65)80255-8. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Intracytoplasmic membranes in Escherichia coli. J Bacteriol. 1966 Sep;92(3):780–783. doi: 10.1128/jb.92.3.780-783.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonington O. G., Pettijohn D. E. The folded genome of Escherichia coli isolated in a protein-DNA-RNA complex. Proc Natl Acad Sci U S A. 1971 Jan;68(1):6–9. doi: 10.1073/pnas.68.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITFIELD J. F., MURRAY R. G. The effects of the ionic environment on the chromatin structures of bacteria. Can J Microbiol. 1956 May;2(3):245–260. doi: 10.1139/m56-029. [DOI] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]
- Zusman D. R., Inouye M., Pardee A. B. Cell division in Escherichia coli: evidence for regulation of septation by effector molecules. J Mol Biol. 1972 Aug 14;69(1):119–136. doi: 10.1016/0022-2836(72)90027-7. [DOI] [PubMed] [Google Scholar]