Abstract
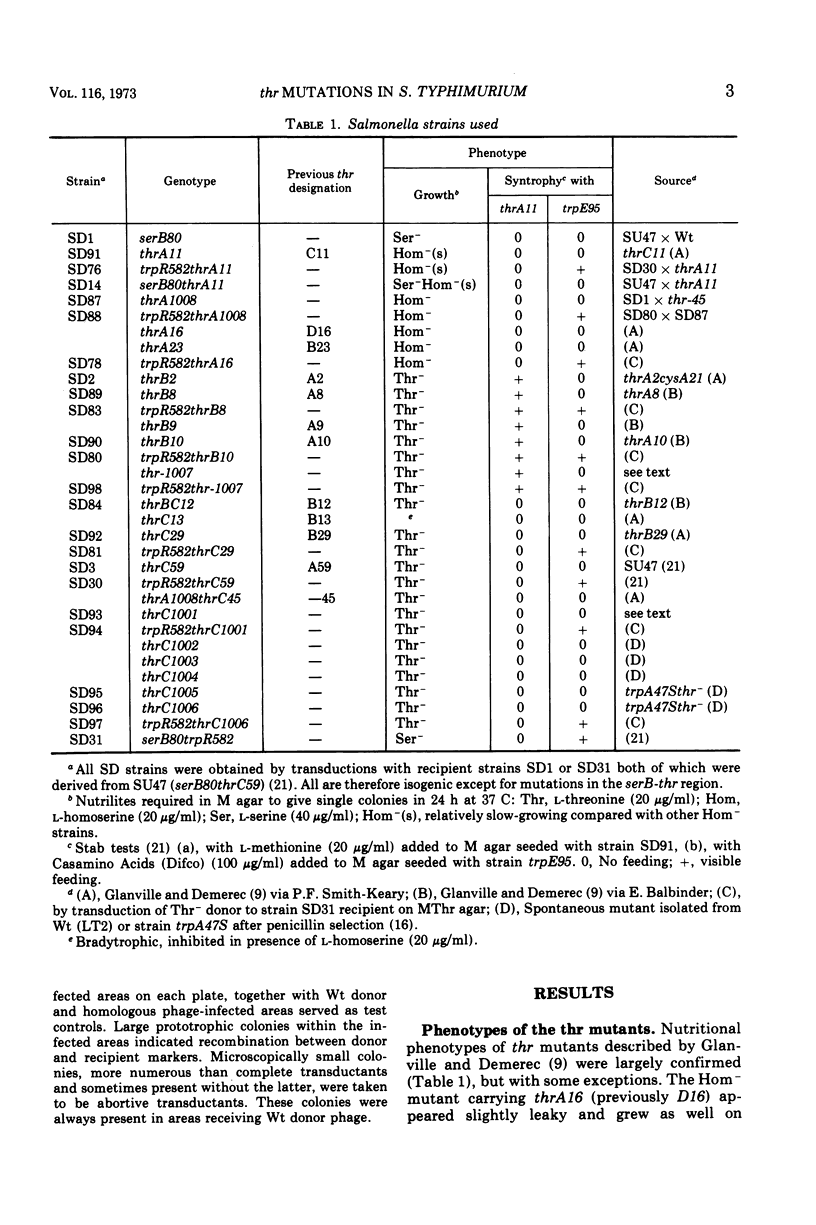
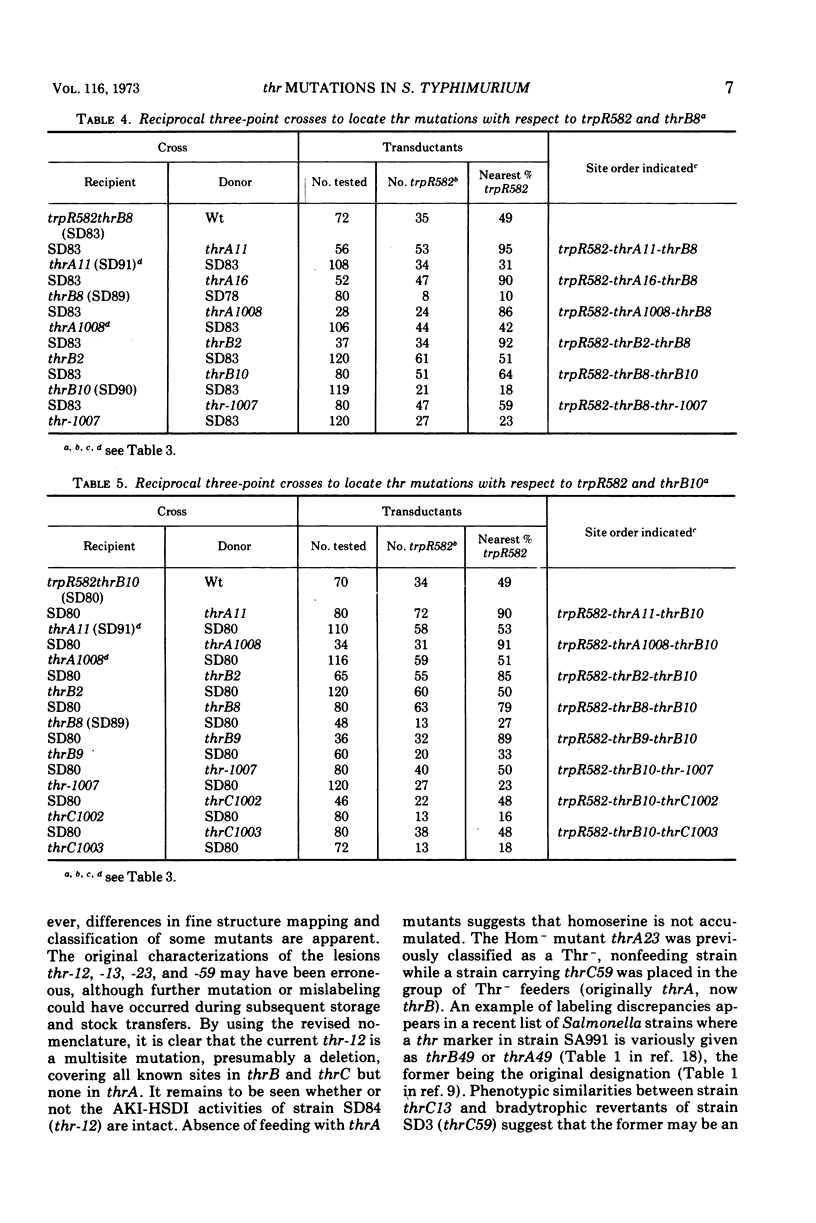
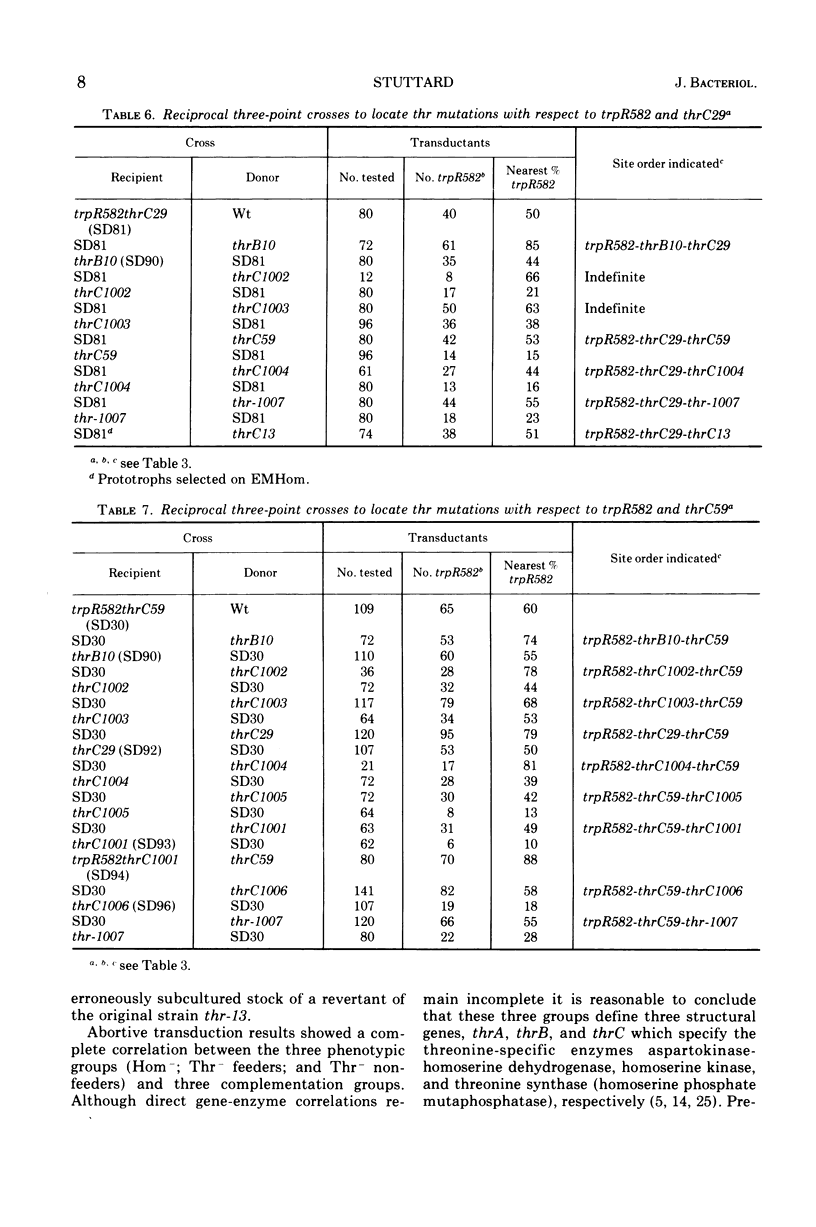
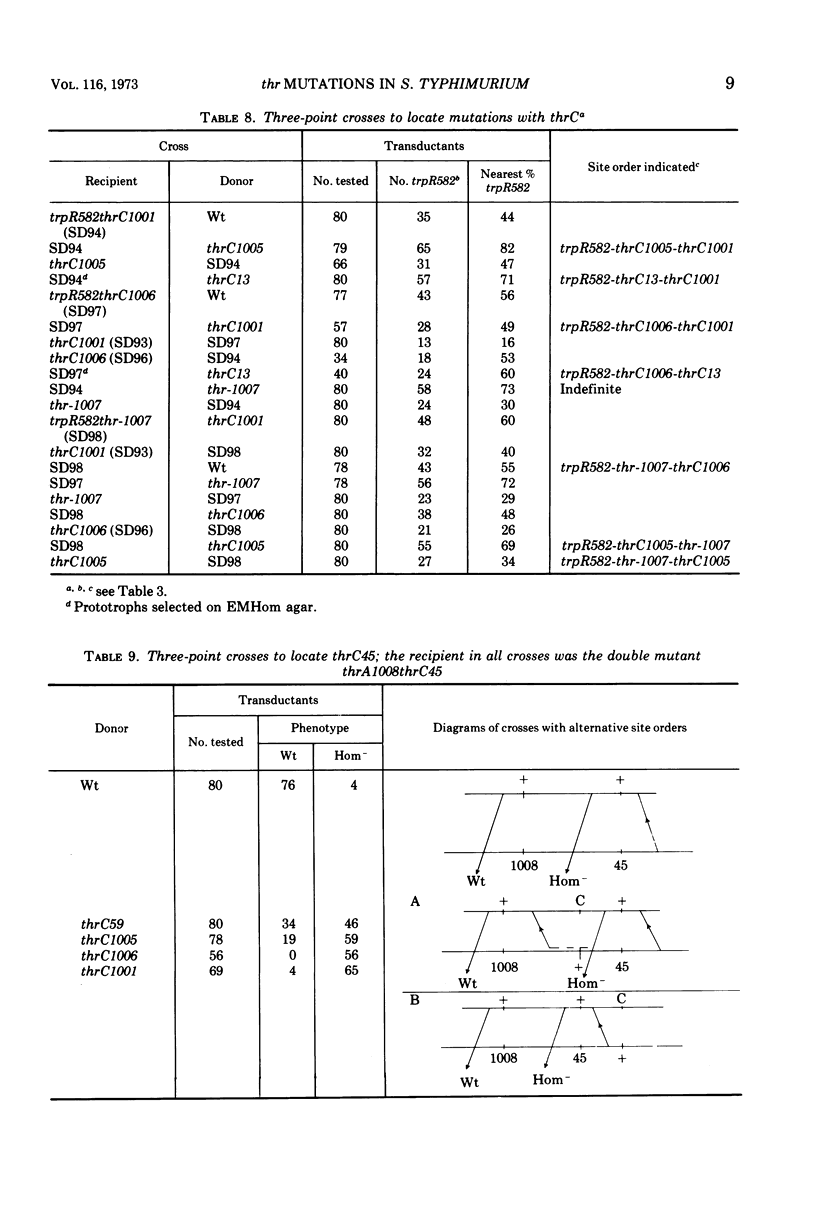
Previous workers divided threonine-requiring (Thr−) strains of Salmonella into three phenotypes with mutations in four complementation groups. The mutations were deemed to define four genes in the order thrD-C-A-B at minute zero on the Salmonella linkage map. In the present study 12 of these mutants were reexamined together with eight new Thr− strains. The three phenotypes were: homoserine-requiring (Hom−); Thr−, feeders of Hom− strains; Thr−, nonfeeders. Exact correlation between these phenotypic groups and three complementation groups was confirmed by abortive transduction. No evidence was found for intergenic complementation between mutations in Hom− strains. It is proposed that thr mutations define three genes rather than four and that these be renamed thrA (Hom−), thrB (Thr− feeders), and thrC (Thr− nonfeeders) to correspond with the sequence of reactions in threonine biosynthesis. Double mutant trpRthr strains were used in reciprocal three-point transduction tests to establish the order of thr mutation sites. Although revisions were made in the classification or location of several mutations, there was an overall correlation of complementation group, phenotype, and map position. The present data provide a basis for further correlation of threonine genes and biosynthetic enzymes, and analysis of cross regulation in aspartate amino acid biosynthesis in Salmonella.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALBINDER E. The fine structure of the loci tryC and tryD of Salmonella typhimurium. I. Determination of the order of mutational sites by three-point transduction tests. Genetics. 1962 Apr;47:469–482. doi: 10.1093/genetics/47.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbinder E. The Fine Structure of the Loci Tryc and Tryd of Salmonella Typhimurium. II. Studies of Reversion Patterns and the Behavior of Specific Alleles during Recombination. Genetics. 1962 May;47(5):545–559. doi: 10.1093/genetics/47.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferata R. L., Freundlich M. Evidence for a methionine-controlled homoserine dehydrogenase in Salmonella typhimurium. J Bacteriol. 1969 Jan;97(1):193–198. doi: 10.1128/jb.97.1.193-198.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferata R. L., Freundlich M. Evidence for channeling of homoserine in Salmonella typhimurium. Biochim Biophys Acta. 1970 Dec 29;222(3):671–674. doi: 10.1016/0304-4165(70)90196-0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Aromatic biosynthesis. VII. Accumulation of two derivatives of shikimic acid by bacterial mutants. J Bacteriol. 1953 Aug;66(2):129–136. doi: 10.1128/jb.66.2.129-136.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcoz-Kelly F., Janin J., Saari J. C., Véron M., Truffa-Bachi P., Cohen G. N. Revised structure of aspartokinase I-homoserine dehydrogenase I of Escherichia coli K12. Evidence for four identical subunits. Eur J Biochem. 1972 Aug 4;28(4):507–519. doi: 10.1111/j.1432-1033.1972.tb01938.x. [DOI] [PubMed] [Google Scholar]
- GORINI L., KAUFMAN H. Selecting bacterial mutants by the penicillin method. Science. 1960 Feb 26;131(3400):604–605. doi: 10.1126/science.131.3400.604. [DOI] [PubMed] [Google Scholar]
- Glanville E V, Demerec M. Threonine, Isoleucine, and Isoleucine-Valine Mutants of Salmonella Typhimurium. Genetics. 1960 Oct;45(10):1359–1374. doi: 10.1093/genetics/45.10.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTMAN P. E., HARTMAN Z., SERMAN D. Complementation mapping by abortive transduction of histidine requiring Salmonella mutants. J Gen Microbiol. 1960 Apr;22:354–368. doi: 10.1099/00221287-22-2-354. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Nass G., Poralla K., Zähner H. Effect of the antibiotic Borrelidin on the regulation of threonine biosynthetic enzymes in E. coli. Biochem Biophys Res Commun. 1969 Jan 6;34(1):84–91. doi: 10.1016/0006-291x(69)90532-4. [DOI] [PubMed] [Google Scholar]
- Riyasaty S., Dawson G. W. The recovery of tryptophan A auxotrophs at high frequency in a strain of Salmonella typhimurium. Genet Res. 1967 Oct;10(2):127–134. doi: 10.1017/s0016672300010867. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Linkage map of Salmonella typhimurium, edition IV. Bacteriol Rev. 1972 Dec;36(4):558–586. doi: 10.1128/br.36.4.558-586.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Ross H., Ziegler L., Mäkelä P. H. F + , Hfr, and F' strains of Salmonella typhimurium and Salmonella abony. Bacteriol Rev. 1972 Dec;36(4):608–637. doi: 10.1128/br.36.4.608-637.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. A. S-amino acid metabolism and its regulation in Escherichia coli and Salmonella typhimurium. Adv Genet. 1971;16:141–165. doi: 10.1016/s0065-2660(08)60357-0. [DOI] [PubMed] [Google Scholar]
- Starnes W. L., Munk P., Maul S. B., Cunningham G. N., Cox D. J., Shive W. Threonine-sensitive aspartokinase-homoserine dehydrogenase complex, amino acid composition, molecular weight, and subunit composition of the complex. Biochemistry. 1972 Feb 29;11(5):677–687. doi: 10.1021/bi00755a003. [DOI] [PubMed] [Google Scholar]
- Stuttard C. Location of trpR mutations in the serB-thr region of Salmonella typhimurium. J Bacteriol. 1972 Aug;111(2):368–374. doi: 10.1128/jb.111.2.368-374.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Véron M., Falcoz-Kelly F., Cohen G. N. The threonine-sensitive homoserine dehydrogenase and aspartokinase activities of Escherichia coli K12. The two catalytic activities are carried by two independent regions of the polypeptide chain. Eur J Biochem. 1972 Aug 4;28(4):520–527. doi: 10.1111/j.1432-1033.1972.tb01939.x. [DOI] [PubMed] [Google Scholar]
- WORMSER E. H., PARDEE A. B. Regulation of threonine biosynthesis in Escherichia coli. Arch Biochem Biophys. 1958 Dec;78(2):416–432. doi: 10.1016/0003-9861(58)90367-9. [DOI] [PubMed] [Google Scholar]


