Abstract
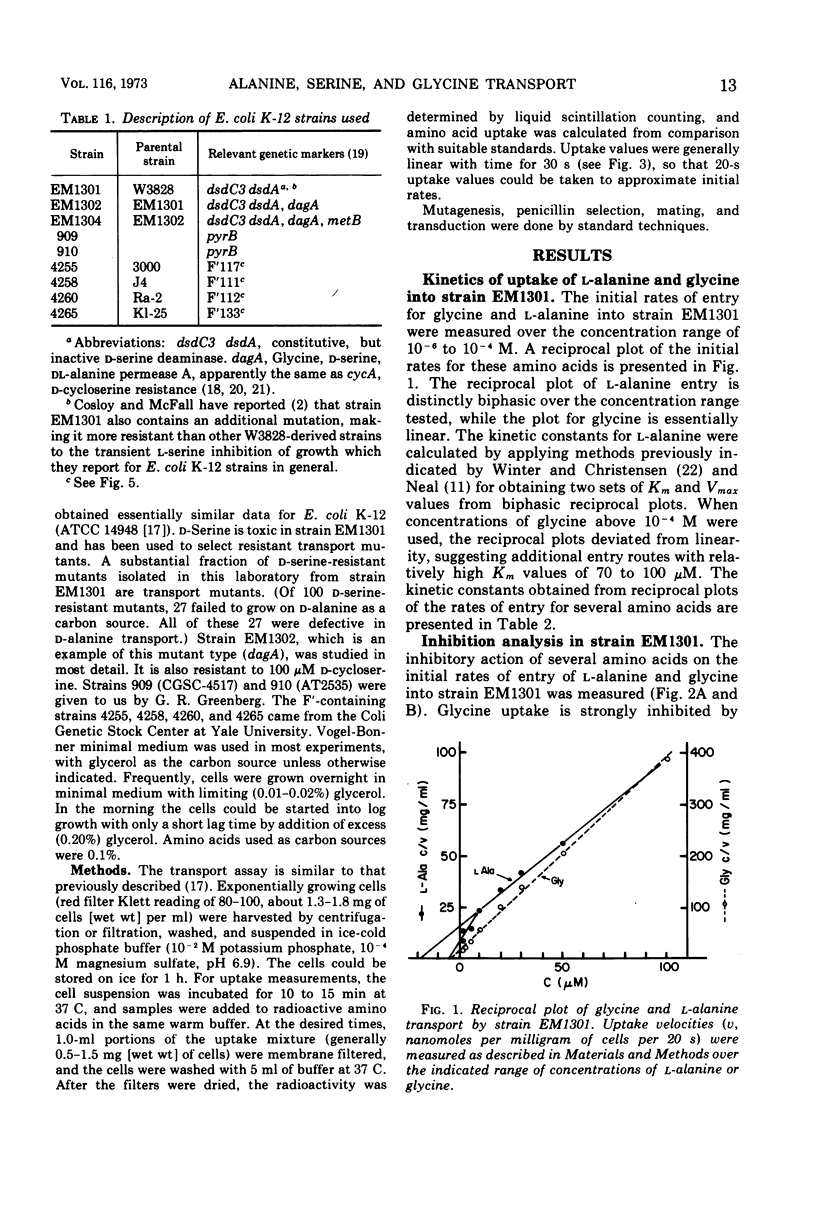
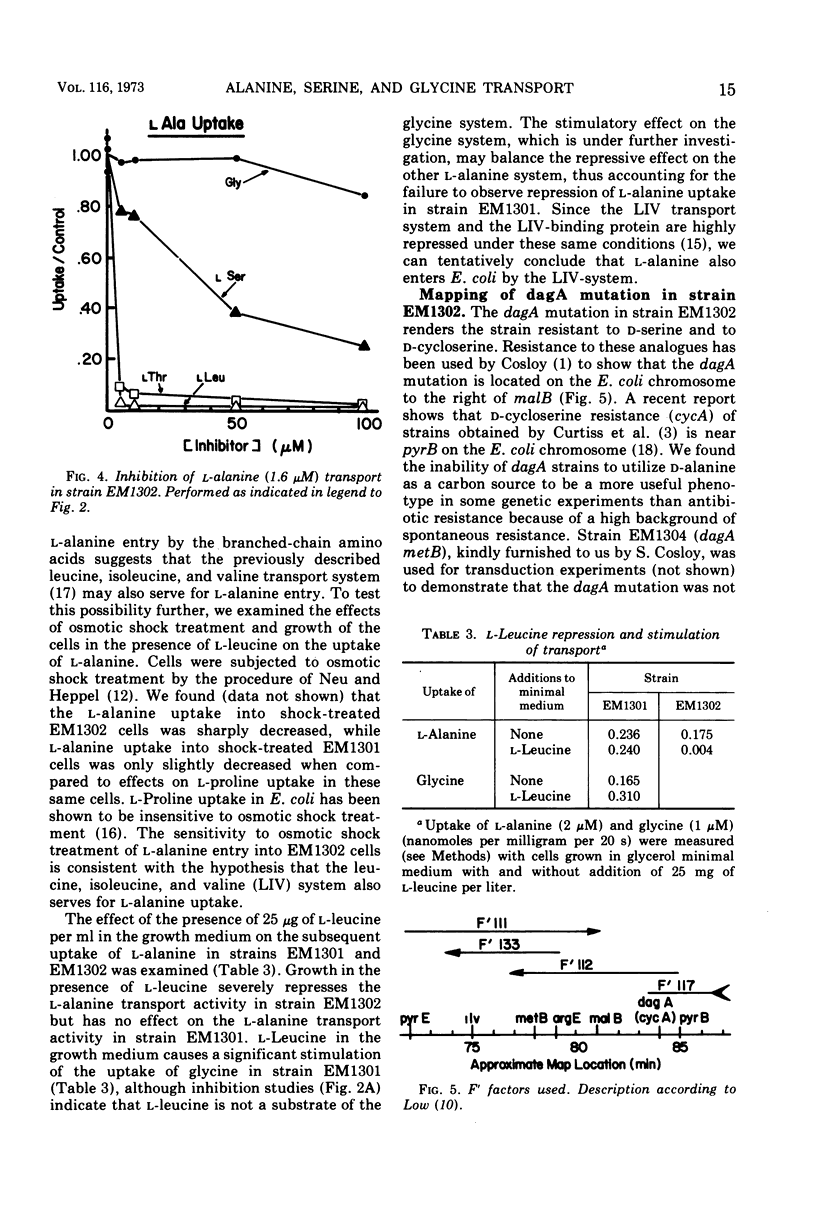
At least two transport systems serve for the entry of alanine, glycine, and serine into Escherichia coli. One of these systems serves mainly for glycine, d-alanine, and d-serine and to some extent for l-alanine, whereas the second serves for l-alanine and perhaps l-serine. These two transport systems have been characterized by kinetic studies and by inhibition analysis. Reciprocal plots for l-alanine entry are distinctly biphasic, giving rise to Km values of about 2 and 27 μM. The major route of glycine entry can be described by a single Km value of about 4 μM. A higher Km value for glycine of around 70 to 100 μM shows that other routes of entry may serve at high concentrations of amino acid. The glycine, d-serine and d-alanine transport system is defective in a d-serine-resistant mutant, strain EM1302. The mutation, dagA, is recessive in dagA/dagA+ merodiploids and is 7 to 12% linked by phage P1 transduction to the pyrB locus of E. coli. E. coli with the dagA mutation are unable to utilize d-alanine as a carbon source, providing an additional basis for selecting such mutants. The remaining l-alanine uptake in dagA mutants is subject to inhibition by l-serine, l-threonine, and l-leucine. It is also sensitive to osmotic shock treatment and repressed by growth of the cells on l-leucine. It appears from a comparison of the properties of the second l-alanine system with those of the leucine, isoleucine, and valine system (LIV system) that the LIV system also serves for the transport of l-alanine and l-threonine and perhaps l-serine.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cosloy S. D. D-serine transport system in Escherichia coli K-12. J Bacteriol. 1973 May;114(2):679–684. doi: 10.1128/jb.114.2.679-684.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosloy S. D., McFall E. Metabolism of D-serine in Escherichia coli K-12: mechanism of growth inhibition. J Bacteriol. 1973 May;114(2):685–694. doi: 10.1128/jb.114.2.685-694.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Charamella L. J., Berg C. M., Harris P. E. Kinetic and genetic analyses of D-cycloserine inhibition and resistance in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1238–1250. doi: 10.1128/jb.90.5.1238-1250.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSEL D., LUBIN M. STABILITY OF ALPHA-HYDROGEN OF AMINO ACIDS DURING ACTIVE TRANSPORT. Biochemistry. 1965 Mar;4:561–565. doi: 10.1021/bi00879a029. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Kostellow A. B. Glycine uptake in Escherichia coli. I. Glycine uptake by whole cells of Escherichia coli W+ and a D-serine-resistant. J Biol Chem. 1968 Apr 10;243(7):1384–1389. [PubMed] [Google Scholar]
- LEVINE E. M., SIMMONDS S. Metabolite uptake by serineglycine auxotrophs of Escherichia coli. J Biol Chem. 1960 Oct;235:2902–2909. [PubMed] [Google Scholar]
- Lombardi F. J., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. 8. The transport of amino acids by membranes prepared from Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):7844–7857. [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal J. L. Analysis of Michaelis kinetics for two independent, saturable membrane transport functions. J Theor Biol. 1972 Apr;35(1):113–118. doi: 10.1016/0022-5193(72)90196-8. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Penrose W. R., Nichoalds G. E., Piperno J. R., Oxender D. L. Purification and properties of a leucine-binding protein from Escherichia coli. J Biol Chem. 1968 Nov 25;243(22):5921–5928. [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino acid transport systems in Escherichia coli K-12. J Biol Chem. 1968 Nov 25;243(22):5914–5920. [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino-acid-binding protein released from Escherichia coli by osmotic shock. J Biol Chem. 1966 Dec 10;241(23):5732–5734. [PubMed] [Google Scholar]
- Russell R. R. Mapping of a D-cycloserine resistance locus in escherichia coli K-12. J Bacteriol. 1972 Aug;111(2):622–624. doi: 10.1128/jb.111.2.622-624.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargel R. J., Hadur C. A., Neuhaus F. C. Mechanism of D-cycloserine action: transport mutants for D-alanine, D-cycloserine, and glycine. J Bacteriol. 1971 Mar;105(3):1028–1035. doi: 10.1128/jb.105.3.1028-1035.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargel R. J., Shadur C. A., Neuhaus F. C. Mechanism of D-cycloserine action: transport systems for D-alanine, D-cycloserine, L-alanine, and glycine. J Bacteriol. 1970 Sep;103(3):778–788. doi: 10.1128/jb.103.3.778-788.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter C. G., Christensen H. N. Contrasts in neutral amino acid transport by rabbit erythrocytes and reticulocytes. J Biol Chem. 1965 Sep;240(9):3594–3600. [PubMed] [Google Scholar]


