Abstract
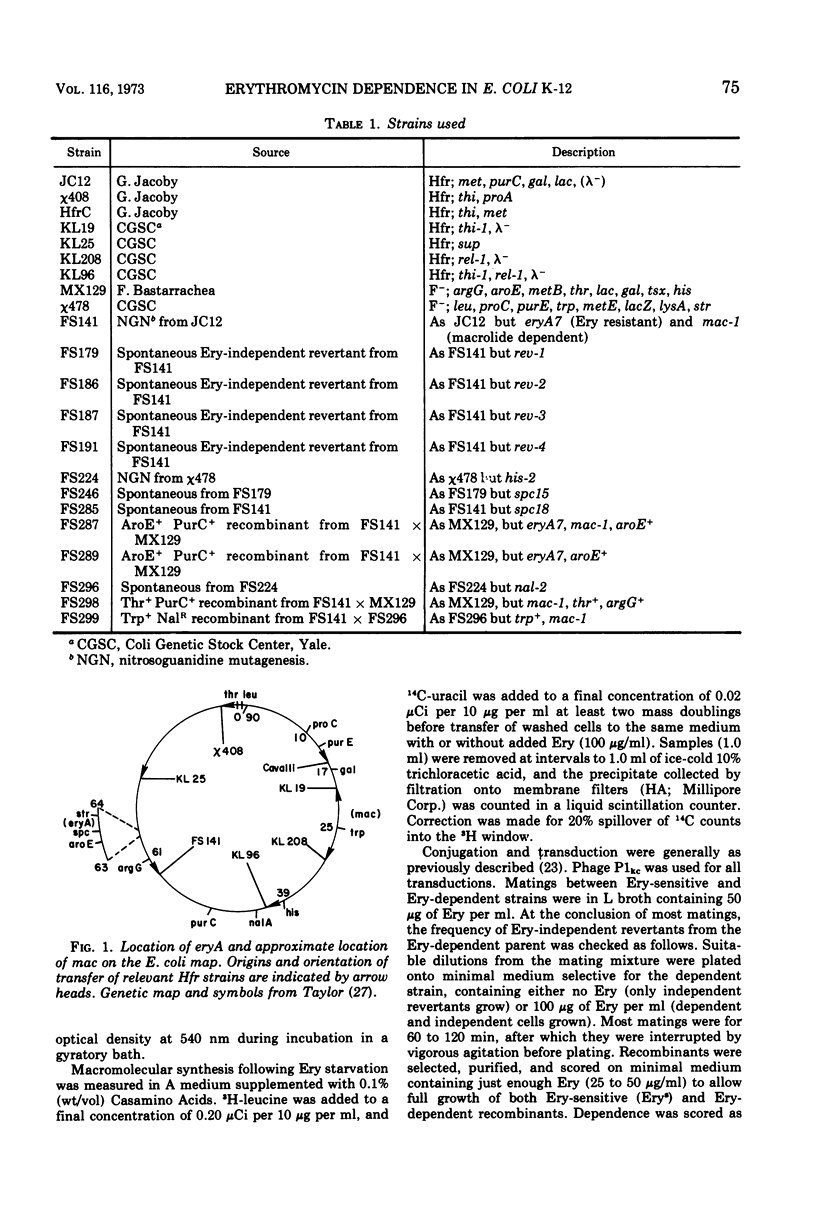
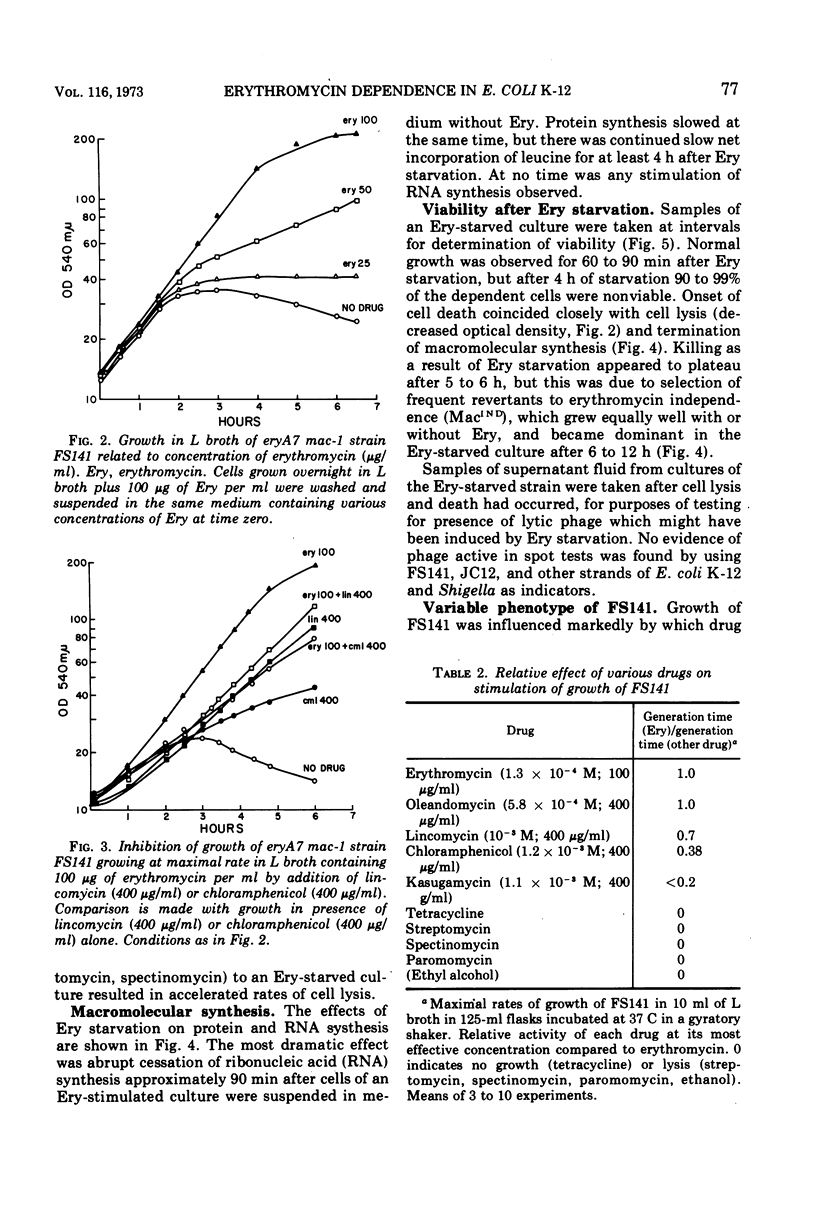
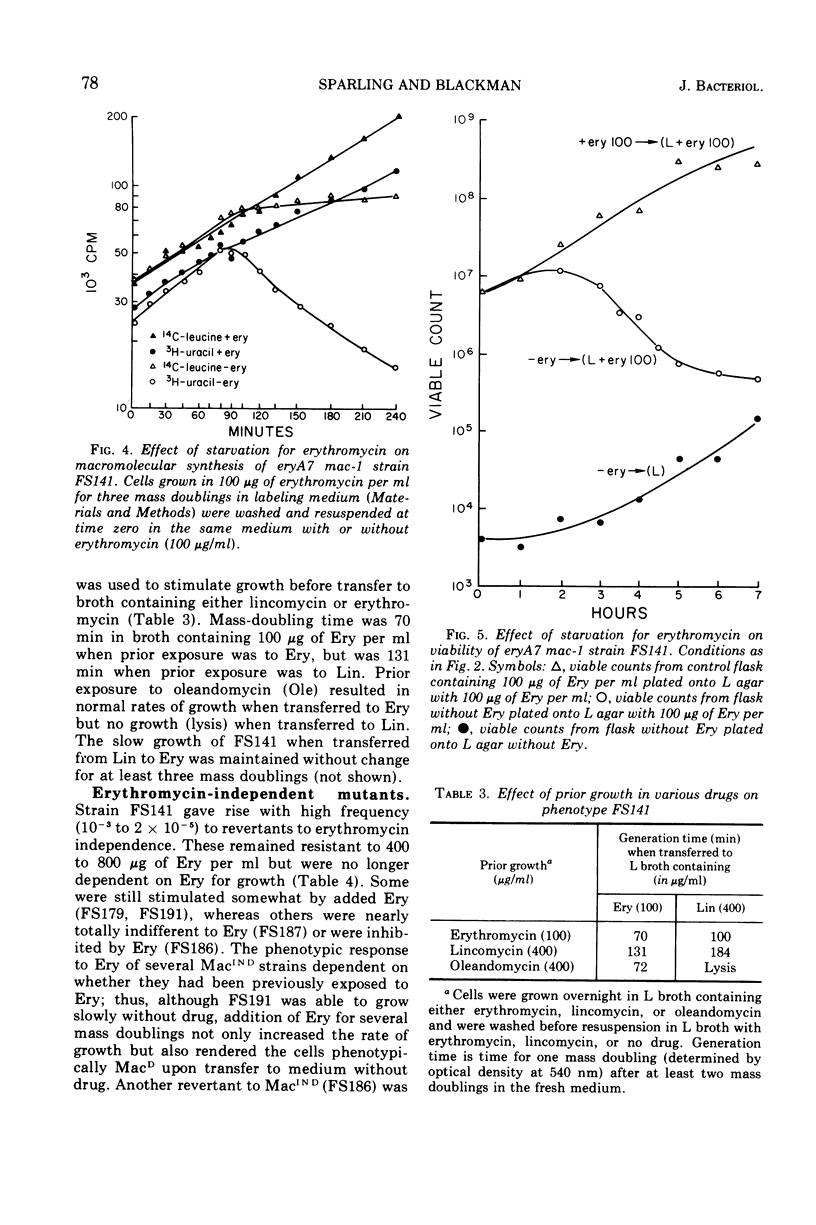
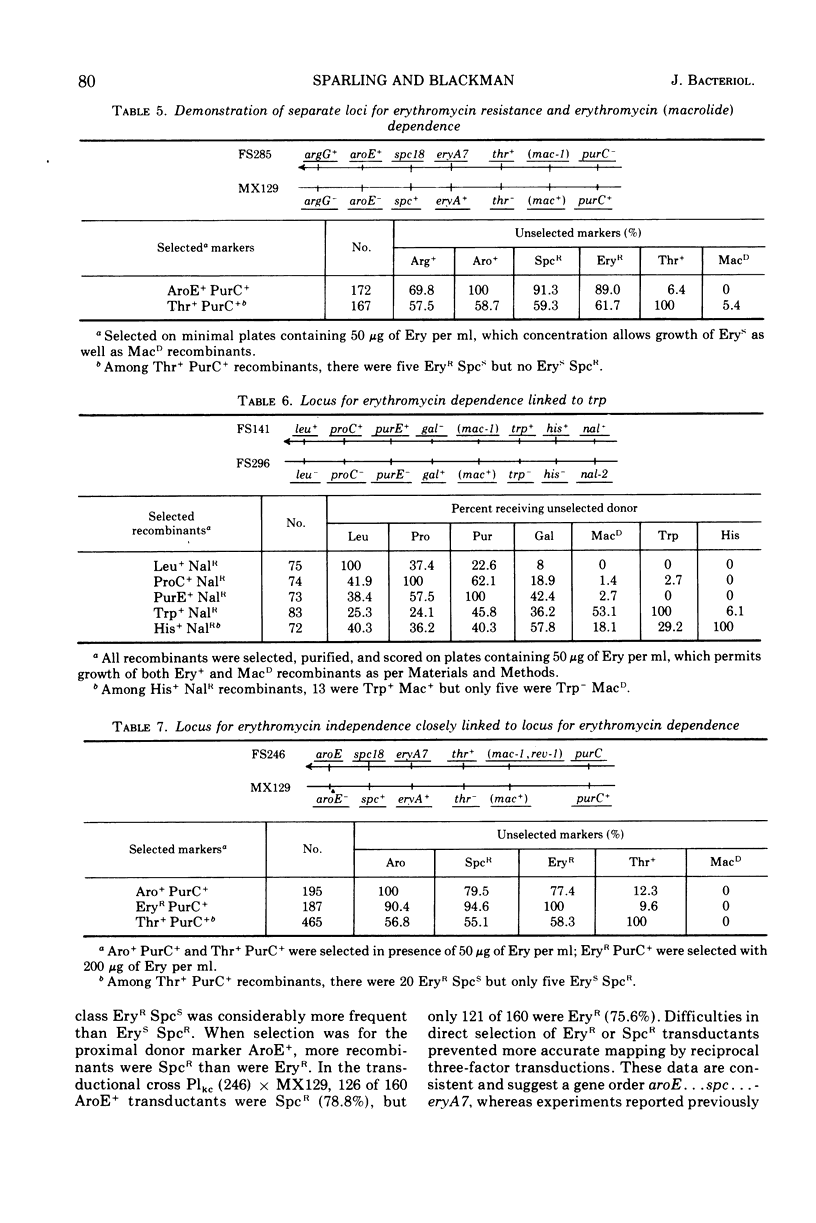
A nitrosoguanidine-induced mutant of Escherichia coli K-12 strain JC12 was absolutely dependent on erythromycin or related macrolide antibiotics for growth. The only other drugs which permitted growth (lincomycin and chloramphenicol) are, like the macrolides, inhibitors of the 50S ribosome. The order of relative effectiveness of these drugs was macrolides > lincomycin > chloramphenicol. Rates of growth with all drugs were concentration dependent. Erythromycin starvation was followed by normal rates of increase in cell mass and macromolecular synthesis for approximately one mass-doubling time, after which macromolecular synthesis abruptly ceased and cell lysis and death occurred. The dependent mutant gave rise spontaneously to revertants to independence with very high frequency (10−4). The gene (mac) for macrolide dependence is located near minute 25 on the E. coli chromosome; it does not result in increased resistance to these drugs. A separate gene for erythromycin resistance (eryA) is located in the cluster of ribosomal structural genes near spc, close to minute 63. Dependence on macrolides was most clearly evident in strains carrying mutations at both eryA and mac.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apirion D., Phillips S. L., Schlessinger D. Approaches to the genetics of Escherichia coli ribosomes. Cold Spring Harb Symp Quant Biol. 1969;34:117–128. doi: 10.1101/sqb.1969.034.01.018. [DOI] [PubMed] [Google Scholar]
- Apirion D. Three genes that affect Escherichia coli ribosomes. J Mol Biol. 1967 Dec 14;30(2):255–275. [PubMed] [Google Scholar]
- Cundliffe E., McQuillen K. Bacterial protein synthesis: the effects of antibiotics. J Mol Biol. 1967 Nov 28;30(1):137–146. doi: 10.1016/0022-2836(67)90249-5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Nomura M. The genetics of bacterial ribosomes. Annu Rev Genet. 1972;6:203–234. doi: 10.1146/annurev.ge.06.120172.001223. [DOI] [PubMed] [Google Scholar]
- Dekio S. Genetic studies of the ribosomal proteins in Escherichia coli. 7. Mapping of several ribosomal protein components by transduction experiments between different strains of Escherichia coli. Mol Gen Genet. 1971;113(1):20–30. [PubMed] [Google Scholar]
- Dekio S., Takata R., Osawa S., Tanaka K., Tamaki M. Genetic studies of the ribosomal proteins in Escherichia coli. IV. Pattern of the alteration of ribosomal protein components in mutants resistant to spectinomycin or erythromycin in different strains of Escherichia coli. Mol Gen Genet. 1970;107(1):39–49. doi: 10.1007/BF00433222. [DOI] [PubMed] [Google Scholar]
- Fernandez-Munoz R., Monro R. E., Torres-Pinedo R., Vazquez D. Substrate- and antibiotic-binding sites at the peptidyl-transferase centre of Escherichia coli ribosomes. Studies on the chloramphenicol. lincomycin and erythromycin sites. Eur J Biochem. 1971 Nov 11;23(1):185–193. doi: 10.1111/j.1432-1033.1971.tb01607.x. [DOI] [PubMed] [Google Scholar]
- Gorini L., Rosset R., Zimmermann R. A. Phenotype masking and streptomycin dependence. Science. 1967 Sep 15;157(3794):1314–1317. doi: 10.1126/science.157.3794.1314. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Streptomycin Resistance in Escherichia Coli Analyzed by Transduction. Genetics. 1960 Jan;45(1):49–62. doi: 10.1093/genetics/45.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Change in methylation of 16S ribosomal RNA associated with mutation to kasugamycin resistance in Escherichia coli. Nat New Biol. 1971 Sep 1;233(35):12–14. doi: 10.1038/newbio233012a0. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Ishitsuka H., Kaji A. Comparative studies on the mechanism of action of lincomycin, streptomycin, and erythromycin. Biochem Biophys Res Commun. 1969 Oct 22;37(3):499–504. doi: 10.1016/0006-291x(69)90943-7. [DOI] [PubMed] [Google Scholar]
- Imamoto F. Diversity of regulation of genetic transcription. I. Effect of antibiotics which inhibit the process of translation on RNA metabolism in Escherichia coli. J Mol Biol. 1973 Feb 25;74(2):113–136. doi: 10.1016/0022-2836(73)90102-2. [DOI] [PubMed] [Google Scholar]
- Ishitsuka H., Kaji A. Release of tRNA from ribosomes by a factor other than G factor. Proc Natl Acad Sci U S A. 1970 May;66(1):168–173. doi: 10.1073/pnas.66.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Putterman M. The intermolecular complex of erythromycin and ribosome. J Mol Biol. 1969 Sep 14;44(2):347–361. doi: 10.1016/0022-2836(69)90180-6. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Wiegand R. G. Mode of action of macrolides. Biochim Biophys Acta. 1968 Apr 22;157(2):404–413. doi: 10.1016/0005-2787(68)90094-4. [DOI] [PubMed] [Google Scholar]
- Monro R. E., Vazquez D. Ribosome-catalysed peptidyl transfer: effects of some inhibitors of protein synthesis. J Mol Biol. 1967 Aug 28;28(1):161–165. doi: 10.1016/s0022-2836(67)80085-8. [DOI] [PubMed] [Google Scholar]
- Otaka E., Itoh T., Osawa S., Tanaka K., Tamaki M. Peptide analyses of a protein component, 50-8, of 50s ribosomal subunit from erythromycin resistant mutants of Escherichia coli and Escherichia freudii. Mol Gen Genet. 1972;114(1):14–22. doi: 10.1007/BF00268742. [DOI] [PubMed] [Google Scholar]
- Otaka E., Teraoka H., Tamaki M., Tanaka K., Osawa S. Ribosomes from erythromycin-resistant mutants of Escherichia coli Q13. J Mol Biol. 1970 Mar;48(3):499–510. doi: 10.1016/0022-2836(70)90061-6. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Sparling P. F., Ikeya Y., Elliot D. Two genetic loci for resistance to kasugamycin in Escherichia coli. J Bacteriol. 1973 Feb;113(2):704–710. doi: 10.1128/jb.113.2.704-710.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F. Kasugamycin resistance: 30S ribosomal mutation with an unusual location on the Escherichia coli chromosome. Science. 1970 Jan 2;167(3914):56–58. doi: 10.1126/science.167.3914.56. [DOI] [PubMed] [Google Scholar]
- Takata R., Osawa S., Tanaka K., Teraoka H., Tamaki M. Genetic studies of he ribosoml proteis in Escherichaoli. V. Mapp-ing of erythromycin resistance mutations which lea to alteration of a 50s ribosomal protein component. Mol Gen Genet. 1970;109(2):123–130. doi: 10.1007/BF00269648. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Tamaki M., Takata R., Osawa S. Low affinity for chloramphenicol of erythromycin resistant Escherichia coli ribosomes having an altered protein component. Biochem Biophys Res Commun. 1972 Mar 24;46(6):1979–1983. doi: 10.1016/0006-291x(72)90747-4. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Teraoka H., Tamaki M., Takata R., Osawa S. Phenotypes represented by a mutational change in a 50s ribosomal protein component, 50-8, in Escherichia coli. Mol Gen Genet. 1972;114(1):9–13. doi: 10.1007/BF00268741. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P., Nomura M. Streptomycin resistance mutation in Escherichia coli: altered ribosomal protein. Science. 1968 Apr 12;160(3824):198–199. doi: 10.1126/science.160.3824.198. [DOI] [PubMed] [Google Scholar]
- Vazquez D., Monro R. E. Effects of some inhibitors of protein synthesis on the binding of aminoacyl tRNA to ribosomal subunits. Biochim Biophys Acta. 1967 Jun 20;142(1):155–173. doi: 10.1016/0005-2787(67)90524-2. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Vogel T., Zamir A., Elson D. Correlation between the peptidyl transferase activity of the 50 s ribosomal subunit and the ability of the subunit to interact with antibiotics. J Mol Biol. 1971 Sep 14;60(2):339–346. doi: 10.1016/0022-2836(71)90298-1. [DOI] [PubMed] [Google Scholar]


