Abstract
It has been known since antiquity that gender-specific behaviors are regulated by the gonads. We now know that testosterone is required for the appropriate display of male patterns of behavior. Estrogen and progesterone, on the other hand, are essential for female typical responses. Research from several groups also indicates that estrogen signaling is required for male typical behaviors. This finding raises the issue of the relative contribution of these two hormonal systems in the control of male typical behavioral displays. In this review we discuss the findings that led to these conclusions and suggest various genetic strategies that may be required to understand the relative roles of testosterone and estrogen signaling in the control of gender specific behavior.
All animals exhibit sex differences in behavior that are characteristic of the species. Such gender typical behaviors are often used to court mates, to defend territory and other resources, and to procure food for mates and offspring. These sexual dimorphisms in behavior are often innate and can be displayed without prior social experience or training, suggesting that the neural circuits that mediate these behaviors are developmentally hardwired in the brain. Nevertheless, the display of these behaviors is tightly regulated by external sensory cues as well as internal physiological regulators such as hormones. Such dual control ensures that animals engage in these behaviors only at the appropriate time and circumstance. While we have learned much about the sensory and hormonal control of sexually dimorphic behaviors (Morris et al, 2004; Arnold, 2004; Simerly, 2002; Scordalakes et al, 2002; Dulac and Torello, 2003; Axel, 1994), several major issues remain to be resolved. Recent advances in genetic engineering in mice should permit a sophisticated dissection of many of these issues. In this review we focus on how such advances will eventually permit a mechanistic understanding of the spatial and temporal requirements of androgen receptor signaling in male typical behaviors in mice.
Rodents exhibit sexual dimorphisms in many social behaviors. These include qualitative and quantitative differences in patterns of aggression, the scent marking of territory, courtship vocalizations, mating, sexual partner preference, and nursing (Goy and McEwan, 1980; Meisel and Sachs, 1994). Note that throughout this review we use the terms “sexually dimorphic behaviors,” “sex specific behaviors” and “sex typical behaviors” interchangeably. Testosterone appears to be necessary and sufficient for the display of most, if not all, male typical behaviors in rodents (Goy and McEwan, 1980; Meisel and Sachs, 1994; Burns-Cusato et al, 2004; Gandelman, 1980; Beeman, 1947). The sensory and hormonal regulation of sexually dimorphic behaviors in various vertebrate species has been exhaustively reviewed elsewhere (Perkins and Roselli, 2007; Pfaus and Heeb, 1997; Kollack-Walker and Newman, 1995; Heeb and Yahr, 1996; Wade and Arnold, 2004), and we limit our discussion to the hormonal control of mating and aggression in male mice. Testosterone is required in a reversible manner in adult rodents for the display of male typical behaviors. Mice and other rodents castrated as adults rapidly lose male typical mating and aggressive patterns of behavior. These deficits, however, can be reversed by supplementation with testosterone (Table 1) (Wallis and Luttge, 1975; Luttge et al, 1974; Edwards and Burge, 1971a; Edwards, 1969; Beeman, 1947). This adult requirement is also referred to as the “activational role” of testosterone in male specific behavior (Phoenix et al, 1959). In addition to this activational role, testosterone also appears to be required perinatally in rodents for the subsequent expression of the full complement of male specific behaviors. For example, male mice castrated within a few hours of birth show significant deficits in mating and intermale aggression as adults (Quadagno et al, 1975; Motelica-Heino et al, 1993; Peters et al, 1972). Importantly, these deficits cannot be corrected in adult life with exogenous testosterone (but see also vom Saal et al, 1976). This irreversible effect of testosterone during early life is also referred to as the “organizational role” for testosterone in male behaviors (Phoenix et al, 1959). Testosterone provided perinatally and in adult life also elicits male typical patterns of mating and aggression in female rodents, suggesting that it is sufficient to drive these behaviors (Edwards and Burge, 1971a; Edwards, 1968; Edwards, 1969).
Table 1.
Hormonal rescue of male mating and aggression deficits
| Adult Castrate |
AR−/Y |
ERα−/− |
||||
|---|---|---|---|---|---|---|
| Mating | Aggression | Mating | Aggression | Mating | Aggression | |
| Testosterone | fulla | fulld | partialf | not tested | partiali,j | nonei |
| DHT | partialb | partiale | noe,f | partiale | partiale,i | partiale |
| Estradiol | partialc | fulle | partiale,f,g | fulle,h | nonee | not tested |
Wallis and Luttge, 1974
Scordlakes and Rissman, 2004
Scordlakes and Rissman, 2003
Testosterone initiates organizational and activational changes in the brain by binding its cognate receptor, the androgen receptor (AR) (Chang et al, 1988; Lubahn et al, 1988). Additionally, testosterone is a prohormone, and it can be metabolized into dihydrotestosterone (DHT) or estrogen (Lephart, 1996; Imperato-McGinley and Zhu, 2002). DHT also binds and activates AR whereas estrogen binds distinct cognate receptors. Estrogen binds to estrogen receptor α (ERα), estrogen receptor β (ERβ), and the G-protein coupled receptor (GPCR), GPR30 (Walter et al, 1985; Kuiper et al, 1996; Revankar et al, 2005). AR and ERα and β are ligand-activated nuclear hormone receptors that modulate the transcription of target genes. By contrast GPR30 is a transmembrane receptor that binds estrogen and initiates heterotrimeric G-protein mediated signaling. While androgens can rapidly activate intracellular signaling cascades independent of direct transcriptional modulation by liganded AR, the functional relevance of these activities for male mating and aggression remains to be elucidated (Michels and Hoppe, 2007; Sun et al, 2006).
Each of the receptors for testosterone and estrogen is expressed in many brain regions, including those that have been implicated in the control of male typical mating and aggression (Shah et al, 2004; Merchenthaler et al, 2004; Simerly et al, 1990; Brailoiu et al, 2007; Lein et al, 2007; Meisel and Sachs, 1994). Such brain regions include the medial amygdala (MeA), the bed nucleus of the stria terminalis (BNST), and the medial preoptic area of the hypothalamus (POA). The enzymes that catalyze the conversion of testosterone into DHT and estrogen are also found in the rodent brain, including in the regions mentioned above (Lauber and Lichtensteiger, 1994; Wagner and Morrell, 1996; Melcangi et al, 1998). This suggests a scenario in which testosterone may also act as a prohormone in the rodent brain (Naftolin et al, 1972). Indeed, estrogen signaling is also required for most male typical behaviors in mice. Adult castrated mice supplemented with estrogen show recovery of most components of male typical mating and fighting (Dalterio et al, 1979; Edwards and Burge, 1971b; Simon and Gandelman, 1978). However, such studies suggest that a complete recovery of male specific patterns of behavior is observed when the castrates are supplemented with both estrogen and DHT (Wallis and Luttge, 1975; Finney and Erpino, 1976). The genetic background is an important factor—in some strains DHT alone can rescue mating and aggression in castrate mice (Luttge et al, 1974; Luttge and Hall, 1973a; Luttge and Hall, 1973b; Burns-Cusato et al, 2004; Maxson et al, 1983). These classic hormone supplementation and deprivation paradigms have provided a strong demonstration of the critical role of androgen and estrogen signaling in male behaviors.
The genetic strategies we discuss in this review should shed additional light on the temporal and spatial requirements of hormone signaling in the control of rodent male behaviors. These two approaches, hormonal manipulations and gene targeting, often provide complementary information. For example, the loss of male mating after adult castration suggests that testosterone is required for this particular behavior. The resumption of male mating following testosterone replacement provides evidence that testosterone is sufficient to initiate these displays. Such studies do not reveal whether testosterone signaling through AR is required for male sexual behaviors nor do they suggest which particular brain regions respond to testosterone to mediate male mating. As genetic deletion of AR also abolishes male mating routines (Ohno et al, 1974; Olsen, 1992; Sato et al, 2004), this approach strongly implicates testosterone signaling through AR in regulating sexual behavior. One limitation of this interpretation is that AR may have a ligand-independent role in modulating male mating. However, as both AR and its cognate ligand, testosterone, are required for male mating, the parsimonious explanation is that testosterone acts via AR to mediate male mating. In other words, a combination of hormonal manipulations and gene targeting often offers a more nuanced insight into the neuroendocrine control of behavior and other physiological processes. We discuss several examples of such a combined approach in the following sections. Note that as the genetic deletion of AR is constitutive this experiment does not reveal when and where AR function is required for male sexual behavior (see Nelson, 1997, for an extended discussion). In later sections, we discuss various genetic approaches that have been devised to generate deletions in a regionally or temporally restricted fashion in order to bypass this limitation.
The role of estrogen signaling in male mating and aggression in mice
Targeted deletion of aromatase, the enzyme that converts testosterone into estrogen, abrogates all estrogen production in the body (Fisher et al, 1998). Male mice null for aromatase have profound deficits in mating and aggression (Table 2) (Honda et al, 1998; Matsumoto et al, 2003; Toda et al, 2001a; Toda et al, 2001b). In a standard mating assay, these mutant males mount a female less frequently than wildtype males, exhibit reductions in intromissions, and rarely ejaculate. Aromatase mutant males also exhibit a severe reduction in aggression towards wildtype males in standard resident-intruder assays. These observations directly implicate a role for estrogen synthesis in the control of male typical behaviors.
Table 2.
Deficits in mating and aggression in male mice mutant for sex steroid receptors or aromatase
| AR−/Y | ERα−/− | ERβ−/− | ERαβ−/− | Aromatase−/− | |
|---|---|---|---|---|---|
| Mount Frequency | nonea,b | decreasede,f,g | unchangedh | nonei | decreasedj,k |
| Intromission Frequency | nonea,b | decreasede,f,g | unchangedh | nonei | decreasedj,k |
| Ejeculation Frequency | nonea,b | nonee,f,g | unchangedh | nonei | decreasedj,k |
| Aggression Towards Males | nonea,b | decreasede,f,g | unchangedh | decreasedi | decreasedj,k |
| Attacked by Resident Males | noc,d | yesc | not tested | not tested | not tested |
Adult males null for ERβ appear to exhibit wildtype levels of mating and aggression (Ogawa et al, 1999; Temple et al, 2003). Male mice homozygous null for ERα on the other hand do exhibit partial deficits in mounting, intromission, and ejaculation in standard tests of sexual behaviors (Ogawa et al, 1997; Wersinger et al, 1997; Ogawa et al, 1998). Note however that these deficits stand in stark contrast to the abrogation of mating observed in adult castrates, suggesting that multiple hormonal mechanisms control male mating. Consistent with this notion, there is a complete loss of all male typical mating behavior in mice homozygous null for both ERα and β (Ogawa et al, 2000). This more profound mating deficit in the ERα and β double mutants suggests a functional redundancy between ERα and ERβ in the control of male sexual behavior. In contrast to this functional redundancy in the control of male mating behavior, intermale aggression appears to require only a functional ERα as ERα mutant males display minimal levels of intermale aggression, a phenotype that strongly resembles the deficits observed in castrates. Taken together, these observations suggest that estrogen signaling underlies the appropriate expression of many components of male mating and aggression.
Note that the mating deficits observed in males doubly mutant for ERα and β are more severe than those in males lacking aromatase. What might account for the difference in phenotypes between males unable to synthesize estrogen and those unable to bind estrogen using nuclear hormone receptors? One possible explanation for this discrepancy is that the ERs have estrogen-independent activities that also control various components of male mating behavior. Alternately, the estrogen deficiency in aromatase null animals may be partially rescued by dietary estrogen. In any event, these genetic studies demonstrate that estrogen signaling via the nuclear hormone receptor type ERs is required for male specific patterns of mating and fighting.
The role of androgen signaling in male mating and aggression in mice
A functional AR is essential for masculinization of the external somatic phenotype and of sex typical behavioral displays in many species. Naturally occurring mutations in AR have been described in rats, mice, cattle, and humans (Bardin et al, 1970; Lyon and Hawkes, 1970; Short, 1967; Morris and Mahesh, 1963). In each instance, males bearing a null allele of AR have feminized external genitalia and other secondary sexual characteristics. Such mutants fail to exhibit male typical behaviors characteristic of the species, and in some cases even display feminized behaviors. Studies in human populations reveal a large spectrum of clinical presentations of men with mutations in AR, ranging from a mild feminization of the external phenotype to completely feminized patients who are karyotypically male (XY). This syndrome, referred to as the Androgen Insensitivity Syndrome (AIS) in the clinical literature, provides dramatic evidence of the influence exerted by gonadal steroid hormones on the development of gender typical physical and socio-sexual traits (McPhaul, 2002).
Mice also require an intact AR for the display of male typical patterns of mating and aggression (Ohno et al, 1974; Olsen, 1992). The tfm (testicular feminization) allele is a naturally occurring mutation which leads to a frameshift in the first exon of the mouse AR locus (Charest et al, 1991). This frameshift leads to a prematurely truncated protein, which lacks the DNA- and ligand-binding domains and is therefore likely to be non-functional. As in other species, tfm males have feminized external genitalia (Lyon and Hawkes, 1970). When presented with females in estrus, tfm males exhibit virtually no mounting or other consummatory aspects of male typical mating behavior (Ohno et al, 1974). However, this demasculinization is not accompanied by the feminization of sexual behavior. Tfm males do not display female typical sexual receptivity towards wildtype males (Ohno et al, 1974). This absence of sexual receptivity persists even when AR null mutants are castrated and primed with estrogen and progesterone, a hormonal regimen that induces sexual receptivity in ovariectomized female mice (Sato et al, 2004). This defeminization of sexual behavior is likely mediated by estrogen signaling through ERβ. Castrated ERβ null males primed with estrogen and progesterone show enhanced female typical receptive behavior compared with WT males, indicating that ERβ signaling is necessary for the defeminization of male behavior (Scordalakes et al, 2002). Therefore, the low-to-normal titers of testosterone observed in tfm males may provide sufficient substrate for the neural synthesis of estrogen, which likely activates ERβ to defeminize sexual behavior.
Unlike wildtype male residents, tfm male residents are not aggressive toward wildtype male intruders in the standard resident-intruder assay (Ohno et al, 1974). This deficit in male resident typical behavior also manifests in altered urine marking. Wildtype males appear to mark their home cage by depositing small quantities of urine all over the cage floor (Desjardins et al, 1973). By contrast, wildtype females and tfm males deposit their urine in large pools in a corner of the cage (N. Shah, unpublished observations). The loss of aggression observed in tfm males resembles the deficits in males null for ERα or ERα and β. ER mutant males have not been tested in urine-marking assays. Nevertheless these findings suggest a dual requirement for AR and ER signaling in the control of male typical territorial marking and defense.
So far we have described the behavioral deficits in mice null for ER or AR mediated signaling. Interestingly, AR also appears to be essential for generating cues that permit other conspecifics to recognize the animal as being male. Tfm intruder males do not elicit aggression from resident wildtype males (Ohno et al, 1974). By contrast, ERα null intruder males are recognized as males and are attacked in resident-intruder tests (Scordalakes and Rissman, 2004). Male mice castrated as adults also do not elicit aggression in such testing, consistent with a role for AR in generating male specific cues (Mugford and Nowell, 1970). Adult castrate males are attacked when their backs are swabbed with urine obtained from wildtype male mice, suggesting that AR may regulate the production of male typical pheromonal signatures (Maruniak et al, 1986).
The relative contributions of AR and ER signaling to male mating and aggression
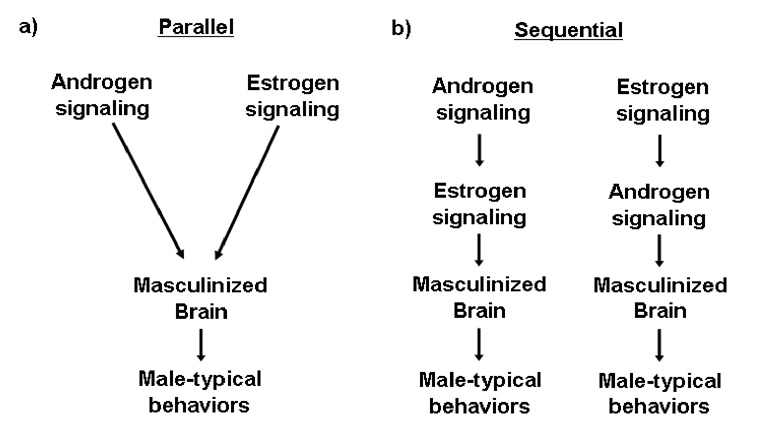
Male mice homozygous null for ERα and β do not engage in male specific patterns of mating and fighting. Similarly, tfm males also do not mate or fight. This dual genetic requirement suggests that these two hormonal systems operate independently to regulate these male specific behaviors (Figure 1). Alternately, the estrogen and androgen signaling systems may act sequentially within the same pathway to regulate male mating and fighting. Note that the models shown in Figure 1 simply provide a genetic framework to understand the relative contributions of AR and ER in the control of male mating and fighting. These models do not reveal the cellular basis for the requirement for AR or ER in these behaviors.
Figure 1. Models for the control of male typical behaviors by androgen and estrogen signaling.
AR and ER may directly regulate male behavior through parallel (a) or sequential (b) pathways in the brain. (a) Androgen and estrogen signaling operate independently of one another to masculinize the brain and behavior. (b) AR and ER may function within the same circuits to masculinize behavior. In this scenario, AR and ER are postulated to interact epistatically, such that either one could function upstream of the other receptor.
The behavioral deficits in mice mutant for AR or ER do not demonstrate that these receptors directly activate mating or fighting by signaling within the neural circuits that mediate these behaviors. It is possible that the phenotype observed in these mutants arises from secondary effects of the deletion. For example, it is formally possible that a group of neurons that participates in the neural circuit that mediates male mating co-expresses both AR and ERα. In this hypothetical scenario, AR may regulate neuronal survival whereas ERα may modulate transcription of a set of genes whose products are required for the neurons to regulate male mating. In tfm males therefore, the abrogation of male sexual behavior could result from the loss of this particular group of neurons. In this instance, crossing the tfm allele into a mouse strain over-expressing an anti-apoptotic gene such as Bcl-2 in these neurons should prevent their cell death, thereby “rescuing” the mating deficit (Zup et al, 2003; Forger et al, 2004). However, males null for ERα will exhibit a mating deficit that cannot be rescued even in the presence of the transgenic Bcl-2. In this example therefore, AR would play a permissive role in mediating male mating as its function does not directly control the activity of the neural circuit for male sexual behavior (cf. differentiation of the spinal nucleus of the bulbocavernosus, Morris et al, 2004). By contrast, ERα signaling would be a pre-requisite for neuronal function during male mating, and consequently this receptor would play an instructive role in this process. In an alternate scenario, it is possible that both AR and ER play instructive roles in regulating mating behavior. For example, if ER signaling regulates AR signaling, and in turn AR regulates genes required for the neurons to participate in the neural circuit, then both AR and ER could be said to mediate mating in an instructive manner. In fact, estrogen signaling has been shown to regulate AR expression in the rat brain during development, consistent with the notion that disruption of ER signaling may mediate some male sex-typical behaviors by modulating AR (McAbee and DonCarlos, 1999). Testosterone or AR have also been shown to regulate the expression of aromatase in several species (Balthazart and Foidart, 1993; Roselli et al, 1987; Veney et al, 2000). While such regulation remains to be demonstrated in the mouse brain, these studies suggest a sequential, instructive role for AR and ER signaling in the control of male behavior (Figure 1b).
The preceding discussion highlights the fact that much needs to be done to sort out the relative contributions of AR and ER signaling in male mating and aggression. Inducible genetic manipulations of AR and ERα and β should resolve many of these outstanding issues. As discussed below, gonadectomy followed by appropriate hormonal supplementation also offers the possibility of revealing potential interactions between the two hormone systems. Such hormonal supplementation studies, which are analogous to inducible transgenic rescue experiments, provide additional insight into the role of gonadal hormones in organizing and activating sex specific behaviors.
Mating, as well as fighting, can be rescued in adult castrated AR null males by supplementation with estrogen (Olsen, 1992; Sato et al, 2004; Scordalakes and Rissman, 2004). It is striking that in males constitutively null for AR, adult administration of estrogen restores inter-male aggression to essentially wildtype levels. Does this mean that hormonal signaling is dispensable in neonatal life for adult displays of aggression? This remains to be tested, as males with a constitutive deletion of AR have normal levels of testosterone neonatally (Sato et al, 2004). In such males, testosterone levels subsequently decline due to atrophy of the testes later in life (Goldstein and Wilson, 1972). In other words, neonatal aromatization of testosterone to estrogen may be required for permitting aggressive displays in the adult mutants. In contrast to the essentially complete rescue of intermale aggression, estrogen treatment of AR mutant males only partially rescues various consummatory components of male typical mating. This suggests that either the hormonal supplementation is inadequate or that there is a neonatal requirement for intact AR signaling for male mating behaviors.
Testosterone or DHT supplementation of adult castrated ERα null males partially rescues mating and aggression (Ogawa et al, 1998; Scordalakes and Rissman, 2003; Sato et al, 2004). This rescue by DHT suggests that the low levels of mating and aggression observed in intact ERα null males are dependent on AR-mediated signaling. Given that mice mutant for both ERα and β exhibit significantly more profound behavioral deficits than those of the single mutants alone, it is also possible that the low levels of mating and aggression present in intact ERα null males are independent of AR signaling. Note that such androgen supplementation experiments have not been performed to date in adult males doubly homozygous null for ERα and β.
The observation that hormonal supplementation can rescue many, and in some cases most, deficits in mating and aggression in male mice bearing mutations in AR or ER provides insight into the mechanisms that underlie these behaviors. For example, the finding that estrogen administration to adult tfm males can rescue intermale aggression in these mutants immediately suggests that the neural pathways that mediate male fighting can differentiate in the absence of functional AR. It is also possible that the estrogen provided to these males activates an alternate neural pathway to regulate aggression, and that this pathway develops normally in the absence of AR function (Figure 1a). In both instances, the supplementation with estrogen may simply be required because of the testicular atrophy observed in tfm males, which would presumably lead to a decline in local, neural synthesis of estrogen. In an alternate scenario, tfm males may have a homeostatic, compensatory upregulation of ER expression or function in regions that can modulate intermale aggression. Such compensation could permit a functional rescue of fighting when the mutant male is supplemented with estrogen. Compensatory mechanisms have been observed in many other processes during development as well as in adult life in many tissues, including the brain (Davis, 2006; Nelson, 1997). For example, skeletal muscle differentiation is under the control of two basic helix-loop-helix transcription factors, MyoD and Myf-5. Mice doubly null for MyoD and Myf-5 fail to form any skeletal muscle (Rudnicki et al, 1993). However, mice mutant only for MyoD exhibit essentially normal muscle differentiation (Rudnicki et al, 1992). Myf-5 is expressed at higher than wildtype levels in MyoD mutants, indicating that the loss of function of MyoD has been compensated for by the up-regulation of Myf-5 (Weintraub, 1993). The rescue of aggression in tfm males with estrogen administration could also reflect true redundancy in the hormonal mechanisms that regulate this behavior. In other words, both AR and ER could function redundantly to mediate male fighting. We wish to operationally define such redundancy as the existence in vivo of two or more mechanisms that subserve the same process in the wildtype state (Thomas, 1993). If these mechanisms are truly redundant, one should observe normal biological function without alteration of activity or expression of the individual components of one pathway when the other is rendered nonfunctional genetically. An example of such redundancy is demonstrated in glial scarring subsequent to neural injury. Astrocytes and other cells in the nervous system migrate towards an injury, proliferate, and clear debris to aid wound healing. Vimentin and glial fibrillary acidic protein (GFAP), the two major intermediate filament proteins of the astrocyte cytoskeleton, appear to be redundant for glial scar formation. Mice singly null for vimentin or GFAP appear to undergo normal wound healing after injury to neural tissue (Pekny et al, 1999). Importantly, neither GFAP nor vimentin appear to be upregulated in these single mutants, suggesting true redundancy rather than homeostatic compensation (Eliasson et al, 1999). By contrast, mice doubly mutant for vimentin and GFAP have defective glial scar formation, accompanied by increased mortality subsequent to the injury (Pekny et al, 1999). Taken together, these observations suggest that vimentin and GFAP participate in glial scarring in a redundant manner. We should point out that mice singly mutant for vimentin or GFAP do have deficits in processes unrelated to glial scar formation (Shibuki et al, 1996; Terzi et al, 1997), providing a good example of redundant function for some but not all biological processes in which these two proteins participate. It is often difficult to distinguish true redundancy from homeostatic compensation, and the term “functional redundancy” could be used to describe the phenotype until the underlying mechanism is understood.
To summarize, the rescue of deficits in mating or fighting in AR and ER mutants with estrogen or testosterone, respectively, may result from one or a combination of several mechanisms. Identifying the mechanism which operates in vivo will require a molecular understanding of the target genes regulated by sex steroid receptors as well as the identification of specific neural circuits that regulate various routines in male typical mating and fighting. In the section that follows we suggest genetic strategies to understand the role of AR in restricted neuronal populations in regulating mating and aggression in male mice. These genetic strategies are also applicable to the estrogen receptors, and provide a powerful complementary approach to the hormonal manipulations discussed above.
Dissecting the temporal and spatial roles of AR in male behavior
As the findings discussed above illustrate, there are several outstanding issues that need to be resolved about the role of AR in regulating mating and aggression in the mouse. It remains to be genetically demonstrated whether AR is required in the brain to mediate male typical behaviors. Alternately, AR may only function in non-neural, peripheral tissues such as the gonads, indirectly regulating behavior through its effects on the neuroendocrine axis. Thus it is possible that the behavioral deficits observed in tfm males result from a disruption of neuroendocrine regulation, rather than from a loss of AR function within the neural circuits that mediate mating and fighting. If AR is indeed required within the brain to control male specific behaviors, is the requirement purely developmental, adult, or both? And finally, which particular subclasses of neurons require AR to control male typical aggression and mating?
It has so far been difficult to distinguish homeostatic compensation for AR deficiency from redundancy in the requirement for either AR or ER mediated signaling in the regulation of male typical behaviors. Engineering an inducible deletion of AR in the adult brain would effectively bypass any developmental compensatory mechanisms. However, even this approach may not distinguish between redundancy and acute homeostatic mechanisms that may be activated in the face of adult AR deficiency. Additional insight into this issue may be afforded by experiments that determine whether AR or ERs function within the same sets of neurons to regulate fighting or mating. Clearly there is a pressing need for experimental manipulation of AR and ER function that can be performed with spatial and temporal precision. The Cre-loxP system (see Box 1) offers a genetically tractable approach to achieve such precise control of AR function. Indeed, over the past five years, several groups have generated different alleles of AR containing loxP-flanked (“floxed”) exons (Figure 2) (De Gendt et al, 2005; Holdcraft and Braun, 2004; Notini et al, 2005; Sato et al, 2004; Yeh et al, 2002). The exons that have been flanked encode the N-terminal activation domain or the DNA binding domain. Deletion of these exons, which occurs when Cre recombinase is provided in trans, leads to a loss-of-function allele of AR. Indeed, when mice bearing such a floxed AR allele are bred with mice expressing Cre recombinase under the control of an ubiquitous promoter, the male progeny bearing both the floxed AR allele and the transgenic Cre recombinase appear to recapitulate the tfm phenotype (De Gendt et al, 2005; Holdcraft and Braun, 2004; Notini et al, 2005; Sato et al, 2004; Yeh et al, 2002). In the section that follows, we present several genetic strategies utilizing the Cre/lox system that will permit the deletion of AR in a spatially and temporally controlled manner.
Cre Recombinase.
The P1 bacteriophage gene Cre encodes Cre recombinase, which recognizes sequence-specific target sites in DNA referred to as loxP sites (Sauer, 1998). Each loxP element is a pseudopalindromic 34 base pair sequence. This asymmetry in the loxP site has practical consequences. The DNA target flanked by loxP sites (“floxed”) in head to tail orientation will be deleted in the presence of Cre. By contrast, a DNA target flanked by two loxP sites in head to head orientation will be inverted. In the former instance, the deletion of the floxed target is accompanied by the loss of a loxP element, leaving behind a single loxP element as a residue of the recombination event. As the loxP element is 34 base pairs long, it occurs only infrequently in long stretches of genomic DNA. In fact, the sequenced mouse genome appears to contain no endogenous loxP sites. This means that one can safely flox DNA target sites in the mouse genome, as the provision of Cre in trans should lead to recombination only at that locus. Note that appropriate placement of loxP sites in the target locus should not interfere with normal gene function. To confirm that gene function has not been altered, mice carrying the floxed allele, but no Cre, must be analyzed as well.
Various versions of Cre are available, including one fused with a fluorescent reporter protein to enable visualization of the Cre fusion protein (Gagneten et al, 1997). In another variant, the activity of Cre recombinase is ligand inducible (see text). Finally, Cre belongs to a class of recombinase proteins which share the property of mediating recombination events at DNA targets flanked by unique recognition sequences. At least one of these, FLPe, is now widely used in place of Cre in the mouse (Branda and Dymecki, 2004). FLPe recognizes frt sites, which are distinct from the loxP sites recognized by Cre recombinase. Consequently, it is now possible to design experiments using both Cre and FLPe to mediate deletions of distinct targets in the genome of the same animal.
Figure 2. Summary of published loxP flanked AR alleles.
a) This schematic depicts the various functional domains of AR, including the N-terminal transactivation domain (dark gray), the DNA binding domain (white), the hinge region (hatched), and the ligand binding domain (light gray). The various exons encoding each of the functional domains are shown using the same schema (b–e). Several groups have generated loxP flanked (“floxed”) AR alleles. The exons that have been floxed include either those that encode the N-terminal transactivation domain (b: Sato et al, 2004; c: Holdcraft and Braun, 2004) or the DNA binding domain (d: Yeh et al, 2002 and DeGendt et al, 2005; e: Notini et al, 2005). Note that the configuration of loxP sites in (c) will result in the inversion of exon 1 rather than a deletion. The loxP sites are denoted as solid arrowheads.
Spatially restricted manipulation of AR function
The tfm mouse lacks AR signaling in all tissues. However, AR is likely to have important roles in diverse tissues, including the gonads, the pituitary, and the nervous system. The behavioral deficits of AR null mice could in principle result from a disruption of AR signaling in the pituitary or from a lack of AR signaling in the brain. Using the Cre-lox system, brain-specific deletions of AR can be generated with the available Cre and floxed AR lines (Figure 3a) (Cinato et al, 2001; Goebbels et al, 2006; Korets-Smith et al, 2004; Tronche et al, 1999; Tsien et al, 1996; Zhu et al, 2001). Deficits in either mating or aggression in such mice would provide a convincing demonstration of a neural requirement of AR in these behavioral routines. If neural AR is indeed necessary for these behaviors, is it required in the neural circuits that regulate the hypothalamic-pituitary-gonadal (HPG) axis, or in circuits that directly control male behaviors? These two (non-mutually exclusive) possibilities can be distinguished by profiling circulating hormone titers. Any dysfunction of the HPG axis could be bypassed by castration and supplementation with testosterone in order to test for additional deficits in the neural circuits that control aggression and mating.
Figure 3. Spatial and temporal control of AR function with Cre recombinase.
(a) To achieve tissue-specific recombination, the Cre transgene is placed under the control of tissue specific regulatory elements. As Cre expression is spatially restricted, excision of the floxed AR allele occurs only in defined regions, leaving behind a single loxP site. (polyA: polyadenylation sequence.)
(b) To obtain spatial and temporal control of recombination, the CreERT2 fusion protein is expressed under the control of tissue specific regulatory elements. In the absence of tamoxifen, CreERT2 is sequestered in the cytoplasm and cannot act on floxed AR alleles in the nucleus. Binding of tamoxifen induces CreERT2 translocation to the nucleus where CreERT2 is now free to recombine the floxed AR allele.
Temporally controlled manipulation of AR function
Classical experiments have shown that hormonal signaling is required both developmentally and in adulthood for male typical behaviors. However, the relative contribution of AR versus ER signaling at various time points has been difficult to establish using traditional hormone supplementation experiments. The advent of temporally controlled Cre systems now provides a novel way to test the necessity of AR signaling at defined time points. In order to distinguish between the developmental and adult roles of AR in controlling male mating and aggression, one has to compare the behavioral consequences of constitutive deletion of AR in the brain to those resulting from the deletion of AR exclusively in the adult. The generation of ligand-activated Cre recombinase offers an elegant, general solution to this problem (Metzger et al, 1995). In the most widely used version, Cre recombinase is fused to the ligand-binding domain of a mutated version of human ERα (CreERT2), which does not recognize endogenous estrogens, but binds tamoxifen with high affinity (Feil et al, 1997). The CreERT2 fusion protein will only enter the nucleus upon administration of tamoxifen, allowing Cre to access loxP sites in the genome (Figure 3b). Cre needs only to be expressed transiently to recombine floxed targets, so tamoxifen can be administered for a short, defined period. Alternative systems for the temporally restricted manipulation of Cre function are also available (Kellendonk et al, 1996; Gossen and Bujard, 2002). At least a few transgenic lines bearing a CreERT2 allele under the control of a brain restricted promoter have been generated (Erdmann et al, 2007; Kuo et al, 2006), and the use of such strains should permit the inducible deletion of neural AR at defined time points.
If AR is required in the adult brain, it is likely that only a subset of the neurons that express AR control mating and aggression. AR is expressed in pools of neurons in diverse brain regions (Shah et al, 2004; Simerly et al, 1990). In order to define the behavioral role of AR in these subsets, it is necessary to delete AR function selectively within such candidate populations. One approach to achieve such regionally and temporally restricted deletion of AR is to stereotactically deliver Cre recombinase using a viral vector. Among the most promising viral vectors are those generated using either lentiviral or adeno-associated virus (AAV) backbones (Miyoshi et al, 1998; Burger et al, 2005). Both viruses are amenable to routine molecular biological manipulations and can be generated in high titers in the laboratory. These viruses can infect post-mitotic cell types, including neurons, and appear to be relatively non-toxic, allowing for long term survival and behavioral analysis of virally transduced animals. Several groups have demonstrated the feasibility of delivering Cre recombinase stereotactically to the adult mouse brain using either a lentiviral or an AAV preparation (Ahmed et al, 2004; Heldt et al, 2001; Thevenot et al, 2003; Kaspar et al, 2002; Pfeifer, 2001; Rajji et al, 2003; Scammell, 2003). As viral vectors are modular, they can be injected into different regions of the brain in adult mice bearing the floxed AR allele, bypassing the need for the generation of multiple region-specific Cre transgenic lines. The use of virally delivered Cre recombinase will permit a facile analysis of how AR in specific brain regions may mediate male typical behaviors.
In this review we have attempted to synthesize the numerous observations on the roles of androgen and estrogen signaling in the control of male mating and fighting in mice. While we have discussed male typical behaviors in terms of mating and aggression, in fact each of these two behaviors consists of distinct subroutines. Additionally these behaviors have motivational and consummatory components (Wersinger and Rissman, 2000; Bodo and Rissman, 2007; Bakker et al, 2002), and for the most part we have limited our discussion to the consummatory aspects. Nevertheless, the Cre/lox reagents we describe will also be useful in understanding the control of each of these individual subroutines by androgen and estrogen signaling.
Acknowledgements
The authors thank members of the Shah lab and Stavros Lomvardas for many thoughtful discussions and for their comments on the manuscript. This work was supported by a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund (NMS), the McKnight Endowment Fund for Neuroscience (NMS), the Sloan Foundation (NMS), a Graduate Research Fellowship from the National Science Foundation (SAJ), a Genentech Graduate Fellowship (JKC), an Achievement Reward for College Scientists Scholarship (JKC), and the National Institutes of Health (R01 NS049488 to NMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed BY, Chakravarthy S, Eggers R, Hermens WT, Zhang JY, Niclou SP, Levelt C, Sablitzky F, Anderson PN, Lieberman AR, Verhaagen J. Efficient delivery of Cre recombinase to neurons in vivo and stable transduction of neurons using adeno-associated and lentiviral vectors. BMC Neurosci. 2004;5:4. doi: 10.1186/1471-2202-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. Sex chromosomes and brain gender. Nat. Rev. Neurosci. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- Axel R. The molecular logic of smell. Sci. Am. 1994;273:154–159. doi: 10.1038/scientificamerican1095-154. [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J. Sexual partner preference requires a functional aromatase (Cyp19) gene in male mice. Horm. Behav. 2002;42:158–171. doi: 10.1006/hbeh.2002.1805. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A. Brain aromatase and the control of male sexual behavior. J. Steroid Biochem. Mol. Biol. 1993;44:521–540. doi: 10.1016/0960-0760(93)90256-v. [DOI] [PubMed] [Google Scholar]
- Bardin CW, Bullock L, Schneider G, Allison JE, Stanley AJ. Pseudohermaphrodite rat: end organ insensitivity to testosterone. Science. 1970;167:1136–1137. doi: 10.1126/science.167.3921.1136. [DOI] [PubMed] [Google Scholar]
- Beeman EA. The effect of male hormone on aggressive behavior in mice. Physiol. Zool. 1947;20:373–405. doi: 10.1086/physzool.20.4.30151969. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur. J. Neurosci. 2007;25:2182–2190. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J. Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Branda CS, Dymecki SM. Talking about a revolution: the impact of site-specific recominbinases on genetic analyses in mice. Dev. Cell. 2004;6:7–28. doi: 10.1016/s1534-5807(03)00399-x. [DOI] [PubMed] [Google Scholar]
- Burger C, Nash K, Mandel RJ. Recombinant adeno-associated viral vectors in the nervous system. Hum. Gene Ther. 2005;16:781–791. doi: 10.1089/hum.2005.16.781. [DOI] [PubMed] [Google Scholar]
- Burns-Cusato M, Scordalakes EM, Rissman EF. Of mice and missing data: what we know (and need to learn) about male sexual behavior. Physiol. Behav. 2004;83:217–232. doi: 10.1016/j.physbeh.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Chang CS, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240:324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- Charest NJ, Zhou ZX, Lubahn DB, Olsen KL, Wilson EM, French FS. A frameshift mutation destabilizes androgen receptor messenger RNA in the Tfm mouse. Mol. Endocrinol. 1991;5:573–581. doi: 10.1210/mend-5-4-573. [DOI] [PubMed] [Google Scholar]
- Cinato E, Mirotsou M, Sablitzky F. Cre-mediated transgene activation in the developing and adult mouse brain. Genesis. 2001;31:118–125. doi: 10.1002/gene.10014. [DOI] [PubMed] [Google Scholar]
- Couse EF, Curtis Hewitt S, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS. Post natal sex reversal in the ovaries of mice lacking estrogen receptors α and β. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- Dalterio S, Bartke A, Butler K. A single injection of 17β-estradiol facilitates sexual behavior in castrated male mice. Horm. Behav. 1979;13:314–327. doi: 10.1016/0018-506x(79)90048-5. [DOI] [PubMed] [Google Scholar]
- Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu. Rev. Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- De Gendt K, Atanassova N, Tan KA, de Franca LR, Parreira GG, McKinnell C, Sharpe RM, Saunders PT, Mason JI, Hartung S, Ivell R, Denolet E, Verhoeven G. Development and function of the adult generation of Leydig cells in mice with Sertoli cell-selective or total ablation of the androgen receptor. Endocrinology. 2005;146:4117–4126. doi: 10.1210/en.2005-0300. [DOI] [PubMed] [Google Scholar]
- Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualized of urinary marking patterns. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- Dulac C, Torello AT. Molecular detection of pheromone signals in mammals, from genes to behaviour. Nat. Reviews Neurosci. 2003;4:551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- Edwards DA. Mice: Fighting by neonatally androgenized females. Science. 1968;161:1027–1028. doi: 10.1126/science.161.3845.1027. [DOI] [PubMed] [Google Scholar]
- Edwards DA. Early androgen stimulation and aggressive behavior in male and female mice. Physiol. Behav. 1969;4:333–338. [Google Scholar]
- Edwards DA, Burge KG. Early androgen treatment and male and female sexual behavior in mice. Horm. Behav. 1971a;2:49–58. [Google Scholar]
- Edwards DA, Burge KG. Estrogenic arousal of aggressive behavior and masculine sexual behavior in male and female mice. Horm. Behav. 1971b;2:239–245. [Google Scholar]
- Eliasson C, Sahlgren C, Berthold CH, Stakeberg J, Celis JE, Betsholtz C, Eriksson JE, Pekny M. Intermediate filament protein partnership in astrocytes. J. Biol. Chem. 1999;274:23996–24006. doi: 10.1074/jbc.274.34.23996. [DOI] [PubMed] [Google Scholar]
- Erdmann G, Schutz G, Berger S. Inducible gene inactivation in neurons of the adult mouse forebrain. BMC Neurosci. 2007;8:63. doi: 10.1186/1471-2202-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem. Biophys. Res. Comm. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Finney HC, Erpino MJ. Synergistic effect of estradiol benzoate and dihydrotestosterone on aggression in mice. Horm. Behav. 1976;7:391–400. doi: 10.1016/0018-506x(76)90010-6. [DOI] [PubMed] [Google Scholar]
- Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc. Natl. Acad. Sci. U S A. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc. Natl. Acad. Sci. U S A. 2004;101:13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagneten S, Le Y, Miller J, Sauer B. Brief expression of a GFP cre fusion gene in embryonic stem cells allows rapid retrieval of site specific genomic deletions. Nucleic Acids Res. 1997;25:3326–3331. doi: 10.1093/nar/25.16.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandelman R. Gonadal hormones and the induction of intraspecific fighting in mice. Neurosci. Biobehav. Rev. 1980;4:133–140. doi: 10.1016/0149-7634(80)90011-1. [DOI] [PubMed] [Google Scholar]
- Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave KA. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis. 2006;44:611–621. doi: 10.1002/dvg.20256. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Wilson JD. Studies on the pathogenesis of pseudohermaphroditism in the mouse with testicular feminization. J. Clin. Invest. 1972;51:1647–1658. doi: 10.1172/JCI106966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Studying gene function in eukaryotes by conditional gene inactivation. Annu. Rev. Genet. 2002;36:153–173. doi: 10.1146/annurev.genet.36.041002.120114. [DOI] [PubMed] [Google Scholar]
- Goy RW, McEwan BS, editors. Sexual Differentiation of the Brain. Cambridge: MIT Press; 1980. [Google Scholar]
- Heeb MM, Yahr P. C-fos immunoreactivity in the sexually dimorphic area of the hypothalamus and related brain regions of male gerbils after exposure to sex-related stimuli or performance of specific sexual behaviors. Neuroscience. 1996;72:1049–1071. doi: 10.1016/0306-4522(95)00602-8. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- Honda S, Harada N, Ito S, Takagi Y, Maeda S. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem. Biophys. Res. Commun. 1998;252:445–449. doi: 10.1006/bbrc.1998.9672. [DOI] [PubMed] [Google Scholar]
- Imperato-McGinley J, Zhu YS. Androgens and male physiology the syndrome of 5 alpha-reductase-2 deficiency. Mol. Cell Endocrinol. 2002;198:51–59. doi: 10.1016/s0303-7207(02)00368-4. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Vissel B, Bengoechea T, Crone S, Randolph-Moore L, Muller R, Brandon EP, Schaffer D, Verma IM, Lee KF, Heinemann SF, Gage FH. Adeno-associated virus effectively mediates conditional gene modification in the brain. Proc. Natl. Acad. Sci. U S A. 2002;99:2320–2325. doi: 10.1073/pnas.042678699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C, Tronche F, Monaghan AP, Angrand PO, Stewart F, Schutz G. Regulation of Cre recombinase activity by the synthetic steroid RU 486. Nucleic Acids Res. 1996;24:1404–1411. doi: 10.1093/nar/24.8.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Korets-Smith E, Lindemann L, Tucker KL, Jiang C, Kabacs N, Belteki G, Haigh J, Gertsenstein M, Nagy A. Cre recombinase specificity defined by the tau locus. Genesis. 2004;40:131–138. doi: 10.1002/gene.20074. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc. Natl. Acad. Sci. U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, Mirzadeh Z, Soriano-Navarro M, Rasin M, Wang D, Shen J, Sestan N, Garcia-Verdugo J, Alvarez-Buylla A, Jan LY, Jan YN. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell. 2006;127:1253–1264. doi: 10.1016/j.cell.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber ME, Lichtensteiger W. Pre- and postnatal ontogeny of aromatase cytochrome P450 messenger ribonucleic acid expression in the male rat brain studied by in situ hybridization. Endocrinology. 1994;135:1661–1668. doi: 10.1210/endo.135.4.7925130. [DOI] [PubMed] [Google Scholar]
- Lein ES, et al. Genome-wide atlas of gene expression in the mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lephart ED. A review of brain aromatase cytochrome P450. Brain Res. Brain Res. Rev. 1996;22:1–26. [PubMed] [Google Scholar]
- Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988;240:327–330. doi: 10.1126/science.3353727. [DOI] [PubMed] [Google Scholar]
- Luttge WG, Hall NR. Androgen-induced agonistic behavior in castrate male Swiss-Webster mice: comparison of four naturally occurring androgens. Beh. Biol. 1973a;8:725–732. doi: 10.1016/s0091-6773(73)80114-2. [DOI] [PubMed] [Google Scholar]
- Luttge WG, Hall NR. Differential effectiveness of testosterone and its metabolites in the induction of male sexual behavior in two strains of albino mice. Horm. Behav. 1973b;4:31–43. [Google Scholar]
- Luttge WG, Hall NR, Wallis CJ. Studies on the neuroendocrine, somatic and behavioral effectiveness of testosterone and its 5α reduced metabolites in Swiss-Webster mice. Physiol. Behav. 1974;13:553–561. [Google Scholar]
- Lyon MF, Hawkes SG. X-linked gene for testicular feminization in the mouse. Nature. 1970;227:1217–1219. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- Maruniak JA, Wysocki CJ, Taylor JA. Mediation of male mouse urine marking and aggression by the vomeronasal organ. Physiol. Behav. 1986;37:655–657. doi: 10.1016/0031-9384(86)90300-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Honda S, Harada N. Neurological effects of aromatase deficiency in the mouse. J. Steroid Biochem. Mol. Biol. 2003;86:357–365. doi: 10.1016/s0960-0760(03)00345-5. [DOI] [PubMed] [Google Scholar]
- Maxson SC, Shrenker P, Vigue LC. Genetics, hormones, and aggression. In: Svare B, editor. Hormones and Aggressive Behavior. New York: Plenum; 1983. pp. 179–196. [Google Scholar]
- McAbee MD, DonCarlos LL. Estrogen, but not androgens, regulates androgen receptor messenger ribonucleic acid expression in the developing male rat forebrain. Endocrinology. 1999;140:3674–3681. doi: 10.1210/endo.140.8.6901. [DOI] [PubMed] [Google Scholar]
- McPhaul MJ. Androgen receptor mutations and androgen insensitivity. Mol. Cell Endocrinol. 2002;198:61–67. doi: 10.1016/s0303-7207(02)00369-6. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Sachs BD. The Physiology of male sexual behavior. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 3–105. [Google Scholar]
- Melcangi RC, Poletti A, Cavarretta I, Celotti F, Colciago A, Magnaghi V, Motta M, Negri-Cesi P, Martini L. The 5alpha-reductase in the central nervous system: expression and modes of control. J. Steroid Biochem. Mol. Biol. 1998;65:295–299. doi: 10.1016/s0960-0760(98)00030-2. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J. Comp. Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- Metzger D, Clifford J, Chiba H, Chambon P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc Natl Acad Sci U S A. 1995;92:6991–6995. doi: 10.1073/pnas.92.15.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels G, Hoppe UC. Rapid actions of androgens. Front. Neuroendocrinol. 2007 doi: 10.1016/j.yfrne.2007.08.004. doi:10.1016/j.yfrne.2007.08.004 (in press) [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM. Development of a self-inactivating lentivirus vector. J. Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nature Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Morris JM, Mahesh VB. Further Observations On The Syndrome, "Testicular Feminization". Am. J. Obstet. Gynecol. 1963;87:731–748. [PubMed] [Google Scholar]
- Motelica-Heino I, Edwards DA, Roffi J. Intermale aggression in mice: does hour of castration after birth influence adult behavior? Physiol. Behav. 1993;53:1017–1019. doi: 10.1016/0031-9384(93)90284-m. [DOI] [PubMed] [Google Scholar]
- Mugford RA, Nowell NW. Pheromones and their effect on aggression in mice. Nature. 1970;226:967–968. doi: 10.1038/226967a0. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by the anterior hypothalamus of adult male and female rats. Endocrinology. 1972;90:295–298. doi: 10.1210/endo-90-1-295. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. The use of genetic “knockout” mice in behavioral endocrinology research. Horm. Behav. 1997;31:188–196. doi: 10.1006/hbeh.1997.1381. [DOI] [PubMed] [Google Scholar]
- Notini AJ, Davey RA, McManus JF, Bate KL, Zajac JD. Genomic actions of the androgen receptor are required for normal male sexual differentiation in a mouse model. J. Mol. Endocrinol. 2005;35:547–555. doi: 10.1677/jme.1.01884. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lubhan DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc. Natl. Acad. Sci. U S A. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Washburn TF, Taylor J, Luhban DB, Korach KS, Pfaff DW. Modifications of testosterone-dependent behaviors by estrogen receptor-α gene disruption in male mice. Endocrinology. 1998;139:5058–5069. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor β gene-deficient (βERKO) male and female mice. Proc. Natl. Acad. Sci. U S A. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. Abolition of male sexual behaviors in mice lacking estrogen receptors α and β (αβERKO) Proc. Natl. Acad. Sci. U S A. 2000;97:14737–14741. doi: 10.1073/pnas.250473597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S, Geller LN, Lai EV. Tfm mutation and masculinization versus feminization of the mouse central nervous system. Cell. 1974;3:235–242. doi: 10.1016/0092-8674(74)90137-8. [DOI] [PubMed] [Google Scholar]
- Olsen KL. Genetic influences on sexual behavior differentiation. In: Gerall AA, Moltz H, Ward IL, editors. Handbook of Behavioral Neurobiology. New York: Plenum Press; 1992. pp. 1–40. [Google Scholar]
- Pekny M, Johansson CB, Eliasson C, Stakeberg J, Wallen A, Perlmann T, Lendahl U, Betsholtz C, Berthold CH, Frisen J. Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J. Cell Biol. 1999;145:503–514. doi: 10.1083/jcb.145.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins A, Roselli CE. The ram as a model for behavioral neuroendocrinology. Horm. Behav. 2007;52:70–77. doi: 10.1016/j.yhbeh.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters PJ, Bronson FH, Whitsett JM. Neonatal castration and intermale aggression in mice. Phys. Behav. 1972;8:265–268. doi: 10.1016/0031-9384(72)90371-x. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Heeb MM. Implications of immediate-early gene induction in the brain following sexual stimulation of female and male rodents. Brain Res. Bull. 1997;44:397–407. doi: 10.1016/s0361-9230(97)00219-0. [DOI] [PubMed] [Google Scholar]
- Pfeifer A, Brandon EP, Kootstra N, Gage FH, Verma IM. Delivery of the Cre recombinase by a self-deleting lentiviral vector: efficient gene targeting in vivo. Proc. Natl. Acad. Sci. U S A. 2001;98:11450–11455. doi: 10.1073/pnas.201415498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Quadagno DM, Wolfe HG, Ho GKW, Goldman BD. Influence of neonatal castration or neonatal anti-gonadotropin treatment on fertility, phallus development, and male sexual behavior in the mouse. Fertil. Steril. 1975;26:939–944. doi: 10.1016/s0015-0282(16)41362-2. [DOI] [PubMed] [Google Scholar]
- Rajji T, Chapman D, Eichenbaum H, Greene R. The role of CA3 hippocampal NMDA receptors in paired associate learning. J. Neurosci. 2006;26:908–915. doi: 10.1523/JNEUROSCI.4194-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Salisbury RL, Resko JA. Genetic evidence for androgen-dependent and independent control of aromatase activity in the rat brain. Endocrinology. 1987;121:2205–2210. doi: 10.1210/endo-121-6-2205. [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, Braun T, Hinuma S, Jaenisch R. Inactivation of MyoD in mice leads to upregulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992;71:383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, Schnegelsberg PNJ, Stead RH, Braun T, Arnold H, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, Nakamichi Y, Yamamoto Y, Nakamura T, Yoshimura K, Yoshizawa T, Metzger D, Chambon P, Kato S. Brain masculinization requires androgen receptor function. Proc. Natl. Acad. Sci. U S A. 2004;101:1673–1678. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B. Inducible gene targeting in mice using the cre/lox system. Methods. 1998;14:381–392. doi: 10.1006/meth.1998.0593. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Arrigoni E, Thompson MA, Ronan PJ, Saper CB, Greene RW. Focal deletion of the adenosine A1 receptor in adult mice using an adeno-associated viral vector. J. Neurosci. 2003;23:5762–5770. doi: 10.1523/JNEUROSCI.23-13-05762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scordalakes EM, Imwalle DB, Rissman EF. Oestrogen's masculine side: mediation of mating in male mice. Reproduction. 2002;124:331–338. doi: 10.1530/rep.0.1240331. [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF. Aggression in male mice lacking functional estrogen receptor α. Behav. Neurosci. 2003;117:38–45. [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor α. Genes Brain Behav. 2004;3:20–26. doi: 10.1111/j.1601-183x.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Shibuki K, Gomi H, Chen L, Bao S, Kim JJ, Wakatsuki H, Fujisaki T, Fujimoto K, Katoh A, Ikeda T, Chen C, Thompson RF, Itohara S. Deficient cerebellar long-term depression, impaired eyeblink conditioning, and normal motor coordination in GFAP mutant mice. Neuron. 1996;16:587–599. doi: 10.1016/s0896-6273(00)80078-1. [DOI] [PubMed] [Google Scholar]
- Short RV. Reproduction. Annu. Rev. Physiol. 1967;29:373–400. doi: 10.1146/annurev.ph.29.030167.002105. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J. Comp. Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Ann. Rev. Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simon NG, Gandelman R. Aggression-promoting and aggression-eliciting properties of estrogen in male mice. Phys. Behav. 1978;21:161–164. [Google Scholar]
- Sun YH, Gao X, Tang YJ, Xu CL, Wang LH. Androgens induce increases in intracellular calcium via a G protein-coupled in LNCaP prostate cancer cells. J. Androl. 2006;27:671–678. doi: 10.2164/jandrol.106.000554. [DOI] [PubMed] [Google Scholar]
- Temple JL, Scordalakes EM, Bodo C, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta gene disrupts pubertal male sexual behavior. Horm. Behav. 2003;44:427–434. doi: 10.1016/j.yhbeh.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Terzi F, Henrion D, Colucci-Guyon E, Federici P, Babinet C, Levy BI, Briand P, Friedlander G. Reduction of renal mass is lethal in mice lacking vimentin. Role of endothelin-nitric oxide imbalance. J. Clin. Invest. 1997;100:1520–1528. doi: 10.1172/JCI119675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenot E, Cote F, Colin P, He Y, Leblois H, Perricaudet M, Mallet J, Vodjdani G. Targeting conditional gene modification into the serotonin neurons of the dorsal raphe nucleus by viral delivery of the Cre recombinase. Mol. Cell Neurosci. 2003;24:139–147. doi: 10.1016/s1044-7431(03)00131-3. [DOI] [PubMed] [Google Scholar]
- Thomas JH. Thinking about genetic redundancy. Trends Genet. 1993;9:395–399. doi: 10.1016/0168-9525(93)90140-d. [DOI] [PubMed] [Google Scholar]
- Toda K, Saibara T, Okada T, Onishi S, Shizuta Y. A loss of aggressive behaviour and its reinstatement by oestrogen in mice lacking the aromatase gene (Cyp19) J. Endocrinol. 2001a;168:217–220. doi: 10.1677/joe.0.1680217. [DOI] [PubMed] [Google Scholar]
- Toda K, Okada T, Takeda K, Akira S, Saibara T, Shiraishi M, Onishi S, Shizuta Y. Oestrogen at the neonatal stage is critical for the reproductive ability of male mice as revealed by supplementation with 17β-oestradiol to aromatase gene (Cyp19) knockout mice. J. Endocrinol. 2001b;168:455–463. doi: 10.1677/joe.0.1680455. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- Veney SL, Rissman EF, Freeman LM. Perinatal organization of a sexually dimorphic aromatase enzyme-containing immunoreactive nucleus. Neuroendocrinology. 2000;11:3409–3412. doi: 10.1097/00001756-200010200-00028. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Gandelman R, Svare B. Aggression in male and female mice: evidence for changed neural sensitivity in response to neonatal but not adult androgen exposure. Phys. Behav. 1976;17:53–57. doi: 10.1016/0031-9384(76)90269-9. [DOI] [PubMed] [Google Scholar]
- Wade J, Arnold AP. Sexual differentiation of the zebra finch song system. Ann. N.Y. Acad. Sci. 2004;1016:540–559. doi: 10.1196/annals.1298.015. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Morrell JI. Distribution and steroid hormone regulation of aromatase mRNA expression in the forebrain of adult male and female rats: a cellular-level analysis using in situ hybridization. J. Comp. Neurol. 1996;370:71–84. doi: 10.1002/(SICI)1096-9861(19960617)370:1<71::AID-CNE7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Wallis CJ, Luttge WG. Maintenance of male sexual behavior by combined treatment with oestrogen and dihydrotestosterone in CD-1 mice. J. Endocrinol. 1975;66:257–262. doi: 10.1677/joe.0.0660257. [DOI] [PubMed] [Google Scholar]
- Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M, Chambon P. Cloning of the human estrogen receptor cDNA. Proc. Natl. Acad. Sci. U S A. 1985;82:7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell. 1993;75:1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor α gene. Horm. Behav. 1997;32:176–183. doi: 10.1006/hbeh.1997.1419. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Rissman EF. Oestrogen receptor alpha is essential for female-directed chemoinvestigatory behaviour but is not required for the pheromone-induced luteinizing hormone surge in male mice. J. Neuroendocrinol. 2000;12:103–110. doi: 10.1046/j.1365-2826.2000.00418.x. [DOI] [PubMed] [Google Scholar]
- Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L, Chang C. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc. Natl. Acad. Sci. U S A. 2002;99:13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, Marth JD, Parada LF. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15:859–876. doi: 10.1101/gad.862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zup SL, Carrier H, Waters EM, Tabor A, Bengston L, Rosen GJ, Simerly RB, Forger NG. Overexpression of Bcl-2 reduces sex differences in neuron number in the brain and spinal cord. J. Neurosci. 2003;23:2357–2362. doi: 10.1523/JNEUROSCI.23-06-02357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]