Abstract
Background and aim: Peak Nasal Inspiratory Flow Rate (PNIFR) is a clinical trial that has been instituted in clinical practice in order to determine the extent of nasal airway patency and it is used to assess the degree of nasal obstruction. This study attempts to provide tables referring to normal values of PNIFR in children and adolescents.
Patients and Methods: Three thousand one hundred and seventy pupils aged between 5 – 18 years, were selected to enter the study. Children with acute or chronic upper airway obstruction, such as acute obstructive pulmonary disease or allergic rhinitis and children below the 3rd percentile for weight and/or height were excluded from the study. All children that took part in the study were subjected to PNIFR measurements by using a portable Youlten Peak Flow meter.
Results: A continuous increase of PNIFR values for boys and girls in relation to age increase was recorded. PNIFR values were higher in boys compared to girls and this difference was statistically significant until the age of 12.
Conclusion: Normal ranges for PNIFR standards are of great importance for the study of nasal patency, evaluation of the degree of nasal obstruction and application of treatment. This is the first time that a detailed description of PNIFR standards becomes available for the Greek population of children and adolescents.
Keywords: nasal inspiratory flow rate, normal values, children, adolescents
Nasal obstruction, may be related to age, body position, rhinic cycle or may be due to the presence of infection (tonsillitis, infection of adenoids), tonsilar or adenoidal hypertrophy, nasal polyps and allergies1. A positive family history should also be considered as an important risk factor of nasal obstruction2. Rhinoscopy is a reliable examination in order to decide the degree of nasal patency. The measurement of Peak Expiratory Flow Rate (PEFR) is a non-specific way to evaluate the presence of obstructive pulmonary disease and its response to treatment. The Peak Nasal Inspiratory Flow Rate (PNIFR) is an objective measurement for nasal obstruction and response to treatment regardless the etiology3–8.
It is a useful test for patients suffering from allergic rhinitis, for establishing the diagnosis and for monitoring treatment efficacy4,9 and furthermore, it is a useful clinical tool to study various environmental factors that may cause nasal obstruction at home or at work10, or for deciding bronchial asthma treatment11.
In this retrospective study, we estimated the normal range of PNIFR values in Greek children according to age and sex and established the standards for Greek children.
Patients – Methods
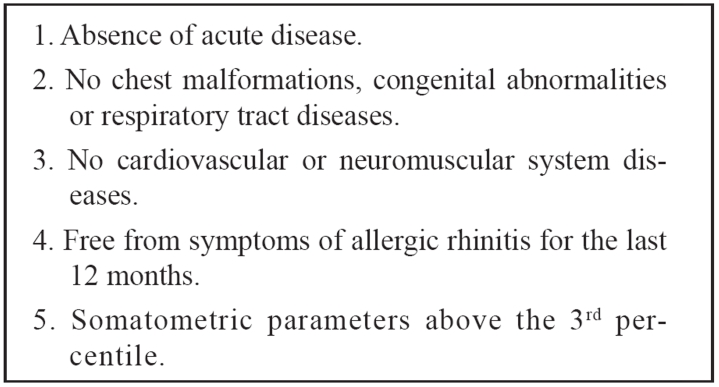
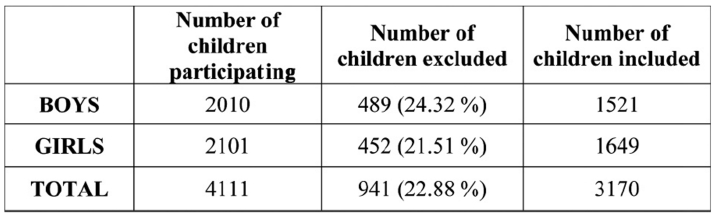
Four thousand one hundred eleven children (2010 boys and 2101 girls) aged between 5-18 years were examined. Children younger than 5 years old were excluded, as they could not follow the instructions. Of them 941 children (489 boys and 462 girls) or 22.88% (24.32% of the boys and 21.51% of the girls) were excluded from the study (Table 1). Eligibility was based on the European Respiratory Society (ERS) criteria for population studies12 (Table 2).
Table 1. Needed criteria to include children and adolescents to the study according to European Respiratory Society (ERS).
Table 2. Number of children who took part, excluded from or included in the statistical analysis of our study according to sex.
Three thousand one hundred and seventy (3170) children (1521 boys and 1649 girls) were selected for the study. Authorities of the primary and high school supported the survey. Parental consensus was obtained. Parents were informed about the study and had to answer a questionnaire 2 or 3 days before their child's clinical examination. Following the recommendations of ISAAC (International Study of Allergy and Asthma in Children)13,14 the questions were simply-worded and understandable, without the use of any medical terms. Each clinical examination took place in a classroom that was properly ventilated and had the appropriate temperature of 22-23℃. Children had to rest for at least 30 minutes before the examination.
The children were dressed with their usual clothes, did not wear shoes and were in upright position when subjected to the test, as suggested in current literature15.
The Seca Model 713 was used for height and weight measurement, as proposed by previous studies16.
PNIFR measurements were performed with a Youlten Peak flow meter9, which is similar to a mini-Wright flow meter. Peak Nasal Inspiratory Flow Rate expressed in L/min is defined as the maximal instantaneous airflow achieved during forced inspiration through the nose. Asking the patient to take a deep, quick forced inspiration after having expired normally, performs the test. The amount of air left in the lungs after a tidal breath out is the Functional Residual Volume (FRV). Then the patient is instructed to inspire deeply through the nose, so that the Total Lung Capacity (TLC) is achieved. Total Lung Capacity (TLC) is the total volume of gas contained in the lungs at the end of a maximal inspiration1, 3. The apparatus function was demonstrated and each pupil was instructed how to inhale forcefully. It is known that the number of attempts a child has to do in order to achieve the best score is inversely proportional to the child's age. Younger children may achieve the best score after the fourth or fifth attempt. The literature indicates that 5 repeated nasal inspirations do not obstruct nose vessels and do not affect the child's score. In our study, all children made 3 attempts of inspiration but only the best score was registered for each child as recommended in the literature 9,17–23. The Youlten Peak flow meter is a rather inexpensive portable device which is easy to use and which should be available, in clinical practice, to all physicians.
Statistical analysis of recorded PNIFR included the estimation of mean, SD, SE and median values corresponding to the 50th percentile value of PNIFR. Curves for the 3rd, 5th, 95th and 97th percentiles values of PNIFR were calculated too24. Analysis of variance (ANOVA) was used to test hypothesis concerning means. Following that, correlation coefficients were calculated and the statistical significance of the Pearson correlation coefficient (R) was separately evaluated for boys, girls and for the total number of subjects. P < 0.05 was considered to be significant.
Results
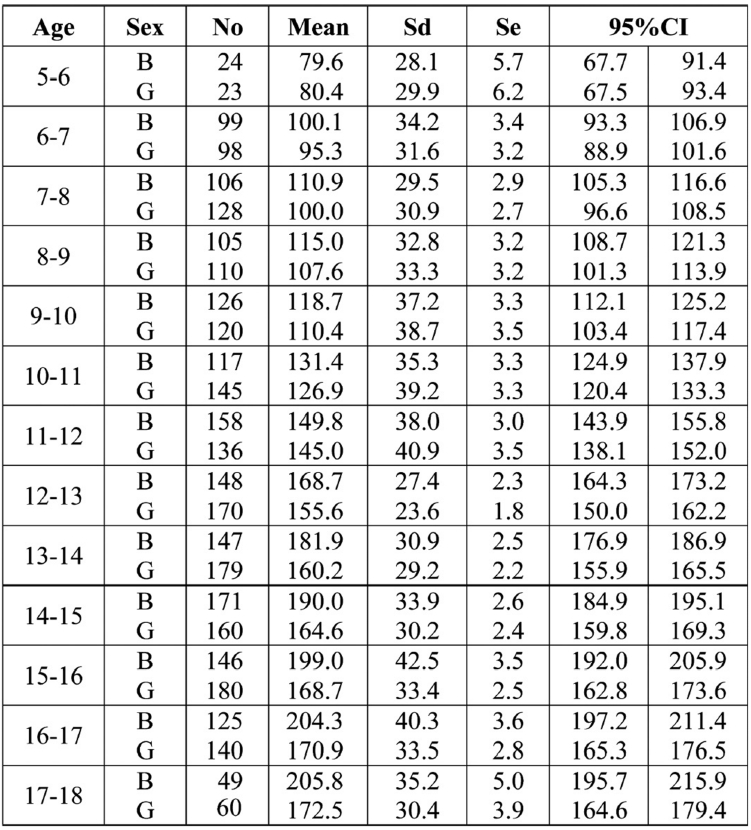
The mean values of PNIFR, SD, SE and 95% confidence intervals for boys and girls according to their age are shown in Table 3.
Table 3. Number of children of each group (No), mean, standard deviation (sd), standard error (se) and the 95% confidence interval (CI) for PNIFR (L/min) for boys (B) and girls (G) according to their age (per year).
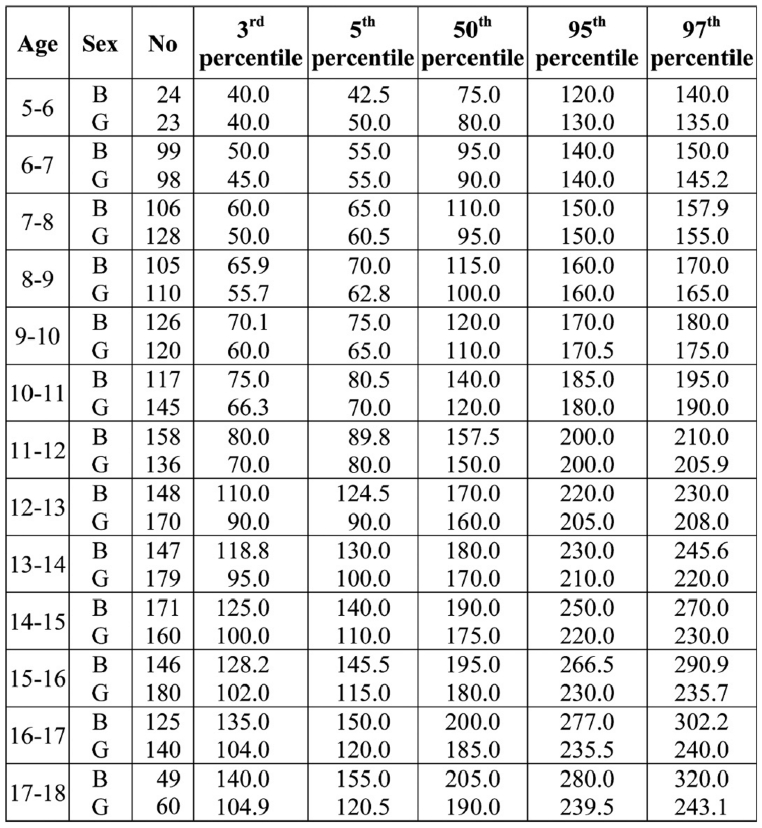
PNIFR values for each percentile for boys and girls in relation to their age are shown in Table 4. A continuous increase of PNIFR values for boys and girls was observed in relation to age increase.
Table 4. Number of children (No) of each group and percentiles for PNIFR (L/min) for boys and girls according to their age (per year).
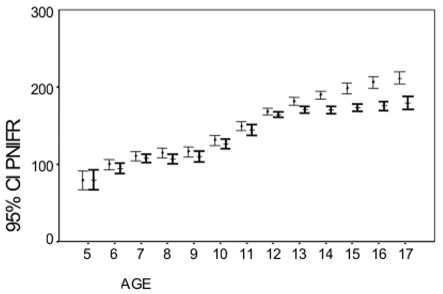
Figure 1 represents the mean PNIFR values for boys and girls when establishing 95% limits of significance. Boys achieved slightly greater scores compared to girls. The difference between boys and girls became statistically significant after the age of 12 (p<0.05).
Figure 1. Mean and 95% confidence interval (CI) for PNIFR according to the age (years) for both sexes (boys I. girls I).
Discussion
Result interpretation, following examinations of the respiratory system, requires the standards of the reference population. Reference of measured variables to population standards will help doctors to decide if there is aberration from the normal. Accurately defined population standards are of great importance.
The term standardized values has been widely used and has substituted the term normal values. The term standardized has been considered as correct since it is very difficult to define accurately the concept of being normal. Measurements characterized as standardized reflect the effort to establish new values and to modify old tables, because the somatometric parameters of the individuals change as time goes by14,25.
Variable deviation from the normal may be attributed to many factors (technological, biological, environmental) 26,27. Technological factors that may alter a result may be attributed to the accuracy of the apparatus, to the cooperation between the doctor and the patient and to the posture of the body and head’s position during the clinical examination26,28. Biological factors affecting the normal values are somatometric parameters such as weight, height and race25–29. Finally, environmental factors may also be considered, such as the region’s altitude, passive smoking and socioeconomic background30.
During the last few years many researchers resorted to the PNIFR for evaluating nasal patency by using Youlten Peak Flow meter and they have proved that this method is reliable4,9–11. Many recent studies have shown that the two techniques are similar, easy to perform and inexpensive18,19,31–33. According to Wihl et al31 repeated PNIFR measurements had a difference of 5 L/min the one from another. Gleeson et al18 similarly to rhinomanometry have also demonstrated high accuracy in PNIFR measurements.
In clinical practice these measurements are used in order to confirm diagnosis of a respiratory tract disease or to monitor treatment efficacy. PNIFR population measurements are considered normally distributed data and the sampling distribution represented in a normal curve can be used to test hypothesis about means. Confidence intervals for each sample mean is the sample mean plus or minus 2 times the standard deviation24,34 for the 95% confidence interval (mean ± 2sd). So, 95% of the normally distributed cases lie within the confidence limits. When a value lies out this confidence interval, then percentiles should be used in order to characterize the result as normal or abnormal.
Our results have shown that PNIFR values tend to increase in proportion with age for boys and girls. There is no statistically significant difference in the mean value of one age group (annual distribution) compared to the next age group between children of the same sex (Graph 1). The increase is in a small range in girls after the age of 13 years, while in boys it keeps increasing in the same range until the age of 17-18 years according to our study. This may be attributed to the fact that girls do not grow taller after the age of 13 years. Other investigators, who found that lung volumes increase in relation to age, reported similar observation. Aivazis et al28 and Barbarousis et al35 reported that lung volumes in girls stop increasing after the age of 14 years and it was attributed to small changes of their height after this age25, 27, 28. Comparing PNIFR values between boys and girls we see that boys, in all age groups, have higher scores compared to girls, with the exception of the 5-6 years age group where PNIFR values for girls are slightly higher compared to those for boys. PNIFR values for boys becomes statistically significantly higher after the age of 12 years (p<0.001).
To our knowledge, there are no references in Greek and international literature concerning PNIFR standards for children until now. All references available concern small groups of adults who had treatment with different drugs, especially those suffering from diseases that affect patency of the upper respiratory track system36–40. We, therefore, consider this study as the first internationally that attempts to assign PNIFR standards for children and adolescents in Greece and to provide an objective measure to all physicians for evaluating nasal airway patency in a relatively simple and inexpensive manner.
References
- 1.Pertuze J, Watson A, Pride NB. Maximum airflow through the nose in humans. J Appl Physiol. 1991;70:1369–1376. doi: 10.1152/jappl.1991.70.3.1369. [DOI] [PubMed] [Google Scholar]
- 2.Fairley JW, Durham LH, Ell SR. Correlation of subjective sensation of nasal patency with nasal inspiratory peak flow rate. Clin Otoloryngol. 1993;18:19–22. doi: 10.1111/j.1365-2273.1993.tb00803.x. [DOI] [PubMed] [Google Scholar]
- 3.Quanjer RhH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R. Lung volumes and forced ventilatory flows. Pneumon. 1993;6:11–43. doi: 10.1183/09041950.005s1693. [DOI] [PubMed] [Google Scholar]
- 4.Youlten LJF. Nasal Airway Patency Measurement in the Assessment of Rhinitis Therapy, Arbeiten aus dem Paul-Ehrlich- Institut, dem Georg-Speyer-Haus und dem Ferdinand-Blum-Institut. New York: Heft 78 Gustav Fisher Verlag, Stuttgart; 1983. pp. 111–113. [PubMed] [Google Scholar]
- 5.Ahlstrom Emanuelson C, Anderson M, Persson CG, Thorsson L, Greiff L. Effects of topical formoterol alone and in combination with budesonide in a pollen season model of allergic rhinitis. Respir Med. 2007 doi: 10.1016/j.rmed.2006.11.017. E pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Can D, Tanac R, Demir E, Gulen F, Veral A. Is the usage of intranasal glucocorticosteroids alone in allergic rhinitis sufficient? Allergy Asthma Proc. 2006;27:248–253. doi: 10.2500/aap.2006.27.2856. [DOI] [PubMed] [Google Scholar]
- 7.Barnes MI, Ward JH, Fardon TC, Lipworth BJ. Effects of levocetirizine as add-on therapy to fluticazone in seasonal allergic rhinitis. Clin Exp Allergy. 2006;36:676–684. doi: 10.1111/j.1365-2222.2006.02478.x. [DOI] [PubMed] [Google Scholar]
- 8.Mim M, Sussman G, Hebert J, Lumry W, Lutsky B, Gates D. Desloratadine therapy for symptoms associated with perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2006;96:460–465. doi: 10.1016/S1081-1206(10)60914-3. [DOI] [PubMed] [Google Scholar]
- 9.Youlten LJF. The peak nasal inspiratory flow meter: a new instrument for the assessment of the response to immunotherapy in seasonal allergic rhinitis. Allergol Immunopathol. 1980;8:344–347. [Google Scholar]
- 10.Nielsen J, Welinder H, Ottosson H, Bensryd I, Venge P, Skerfvings S. Nasal challenge shows pathogenetic relevance of specific IgE serum antibodies for nasal symptoms caused by hexahydrophthalic anhydride. Clin Exp Allergy. 1994;24:440–449. doi: 10.1111/j.1365-2222.1994.tb00932.x. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen S, Hansen OR, Fuglsang G. Influence of inspiratory flow rate upon the effect of a Turbuhaler. Arch Dis Child. 1990;65:308–310. doi: 10.1136/adc.65.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quanjer RhH, Stocks J, Polgar J, Wise M, Karlberg J, Borsboom G. Complication of reference values for lung function measurements in children. Eur Respir J. 1989;(Suppl. 4):184s–261s. [PubMed] [Google Scholar]
- 13.Gratziou Ch, Priftis K, Anagnostakis I, et al. Prevalence of Asthma-Like Symptoms Among Greek Children [abstract] Annual Congress of European Respiratory Society (ERS) 1996:228s. [Google Scholar]
- 14.ISAAC. Steering Committee. Worldwide variations in prevalence of symptoms of asthma, allergic rhinoconjuctivitis and atopic eczema. Lancet. 1998;351:1225–1232. [PubMed] [Google Scholar]
- 15.Ogden C, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and prevention 2000 growth charts for the United States: Improvements to the 1977 National Center for Health statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Aivazis V. Τhe normal child. Standards from 16.700 Greek children 0-17 years [Monography] 1990. [Google Scholar]
- 17.Gardner R, Baker C, Broennle M, et al. American Thoracic Society (ATS). Statement-Snowbird workshop on standardization of spirometry. Am Rev Respir Dis. 1979;119:831–838. doi: 10.1164/arrd.1979.119.5.831. [DOI] [PubMed] [Google Scholar]
- 18.Gleeson MJ, Youlten LJF, Shelton DM, Siodlak MZ, Eiser NM, Wengraf CL. Assessment of nasal airway patency : a comparison of four methods. Clin Otolaryngol. 1986;11:99–107. doi: 10.1111/j.1365-2273.1986.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 19.Holmstrφm M, Scadding GK, Lund VJ, Darby YC. Assessment of nasal obstruction. A comparison between rhinomanometry and nasal inspiratory peak flow. Rhinology. 1990;28:191–196. [PubMed] [Google Scholar]
- 20.Jones AS, Viani L, Phillips D, Charters P. The objective assessment of nasal patency. Clin Otolaryngol. 1991;16:206–211. doi: 10.1111/j.1365-2273.1991.tb01978.x. [DOI] [PubMed] [Google Scholar]
- 21.Clarke RW, Jones AS, Richardson H. Peak nasal inspiratory flow-the plateau effect. J Laryngol Otol. 1995;109:399–402. doi: 10.1017/s0022215100130282. [DOI] [PubMed] [Google Scholar]
- 22.Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. Subjective and objective markers of treatment response in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2000;85:111–114. doi: 10.1016/S1081-1206(10)62449-0. [DOI] [PubMed] [Google Scholar]
- 23.Wilson AM, Dempsey OJ, Sims EJ, Coutie WJR, Paterson MC, Lipworth BJ. Evaluation of treatment response in patients with seasonal allergic rhinitis using domiciliary nasal peak inspiratory flow. Clin Exp Allergy. 2000;30:833–838. doi: 10.1046/j.1365-2222.2000.00749.x. [DOI] [PubMed] [Google Scholar]
- 24.Armitage P, Berry G. Statistical Methods in Medical Research. 2nd ed. Oxford: Blackwell Scientific Publications; 1989. [Google Scholar]
- 25.Carlson JWK, Hoey H, Taylor MRH. Growth and other factors affecting peak expiratory flow rate. Arch Dis Child. 1989;64:96–102. doi: 10.1136/adc.64.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Thoracic Society (ATS) Statement. Lung function testing of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 27.European Respiratory Society (ERS) Lung volumes and forced ventilatory flows. Report Working Party on Standardization of Lung Function Test. Eur Respir J. 1993;6:5–40. [PubMed] [Google Scholar]
- 28.Aivazis V, Hatzimichail A, Stavridis J, Bourli E, Konstantinidis Th, Katsougiannopoulos V. Growth and other factors affecting peak expiratory flow in Greek children. Minerva Pediatrica. 2005;57:83–89. [PubMed] [Google Scholar]
- 29.Cotes JE, Leathart GL. Lung Function Assessment and application in medicine. 5th ed. Blackwell publications; 1993. Lung function throughout life: Determinants and reference values; pp. 445–513. [Google Scholar]
- 30.Paoletti P, Paggiaro PL, Lebowitz MD. Environmental factors in PEF variability. Eur Respir J. 1997;10:64–66. [PubMed] [Google Scholar]
- 31.Wihl JA, Malm L. Rhinomanometry and nasal peak expiratory and inspiratory flow rate. Ann Allergy. 1988;61:50–55. [PubMed] [Google Scholar]
- 32.Nathan Ra, Eccles R, Howarth PH, Steinsvag SK, Togios A. Objective monitoring of nasal patency and nasal physiology in rhinitis. J Allergy Clin Immunol. 2005;115:442–459. doi: 10.1016/j.jaci.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson AM, Sims EJ, Robb F, Cockburn W, Lipworth BJ. Peak inspiratory flow rate is more sensitive than acoustic rhinomanometry or rhinomanometry in detecting corticosteroid response with nasal histamine challenge. Rhinology. 2003;41:16–20. [PubMed] [Google Scholar]
- 34.Trichopoulos D. Medical Statistics. Basic Principles and methods of biomedical statistics. Athens: Parisianos; 1975. [Google Scholar]
- 35.Barbarousis D, Gioulekas D, Aivazis V, Kakavelas H. An investigation of the Lung Function of children and Teenagers of Thessaloniki. Hell Rev Respir Dis. 1987;5:223–236. [Google Scholar]
- 36.Mucha SM, de Tineo M, Naclerio RM, Baroody FM. Comparison of montelukast and pseudoephedrine in the treatment of allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2006;132:164–172. doi: 10.1001/archotol.132.2.164. [DOI] [PubMed] [Google Scholar]
- 37.Barnes ML, Biallosterski BT, Gray RD, Fardon TC, Limorth BJ. Decongestant effects of nasal xylometazoline and mometazone furoate in persistent allergic rhinitis. Rhinology. 2005;43:291–295. [PubMed] [Google Scholar]
- 38.Oldenbeuving NB, Kleinjan A, Mulder PG, et al. Evaluation of an intranasal house dust mite provocation model as a tool in clinical research. Allergy. 2005;60:751–759. doi: 10.1111/j.1398-9995.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- 39.Bhatia S, Baroody FM, de Tineo M, Naclerio RM. Increased nasal airflow with budesonide compared with desloratadine during the allergy season. Arch Otolaryngol Head Neck Surg. 2005;131:223–228. doi: 10.1001/archotol.131.3.223. [DOI] [PubMed] [Google Scholar]
- 40.Lund VJ, Black JH, Szabo LZ, Schrewelius C, Akerlund A. Efficacy and tolerability of budesonide aqueous nasal spray in chronic rhinosinusitis patients. Rhinology. 2004;42:57–62. [PubMed] [Google Scholar]