Abstract
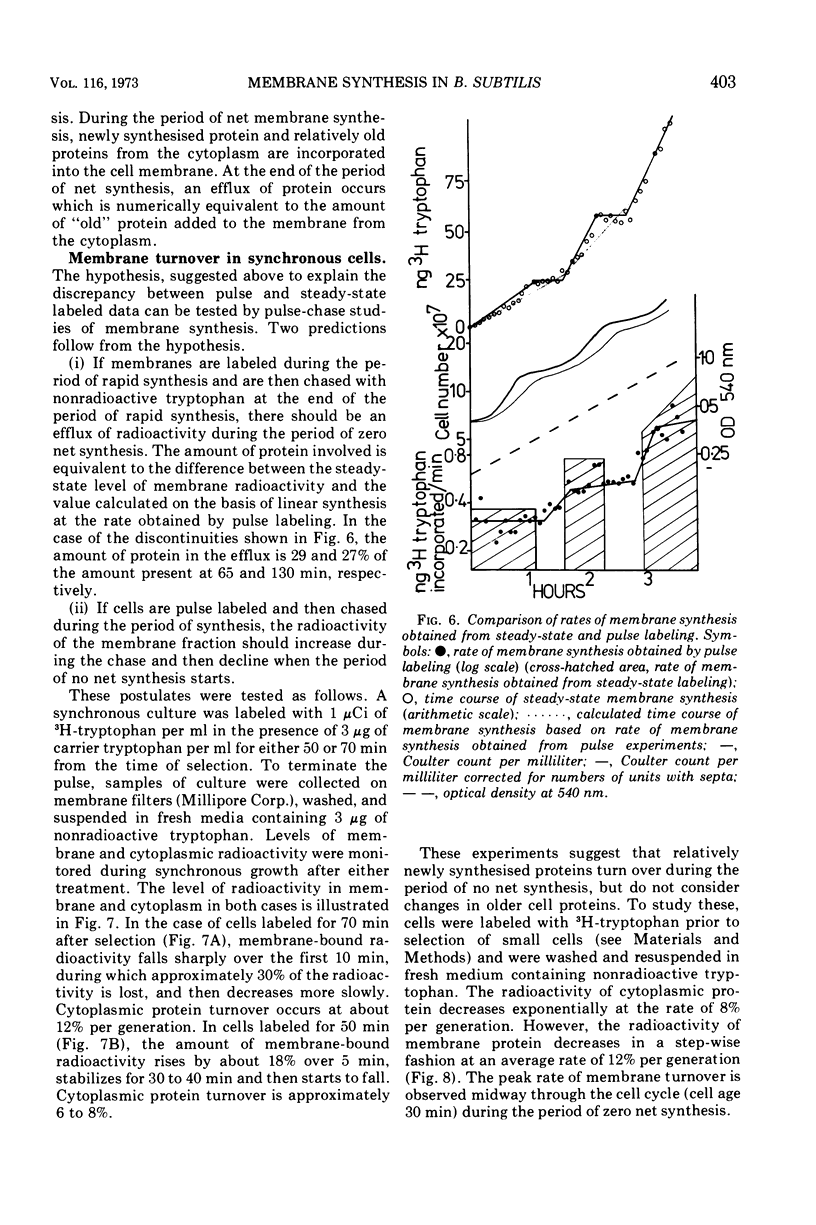
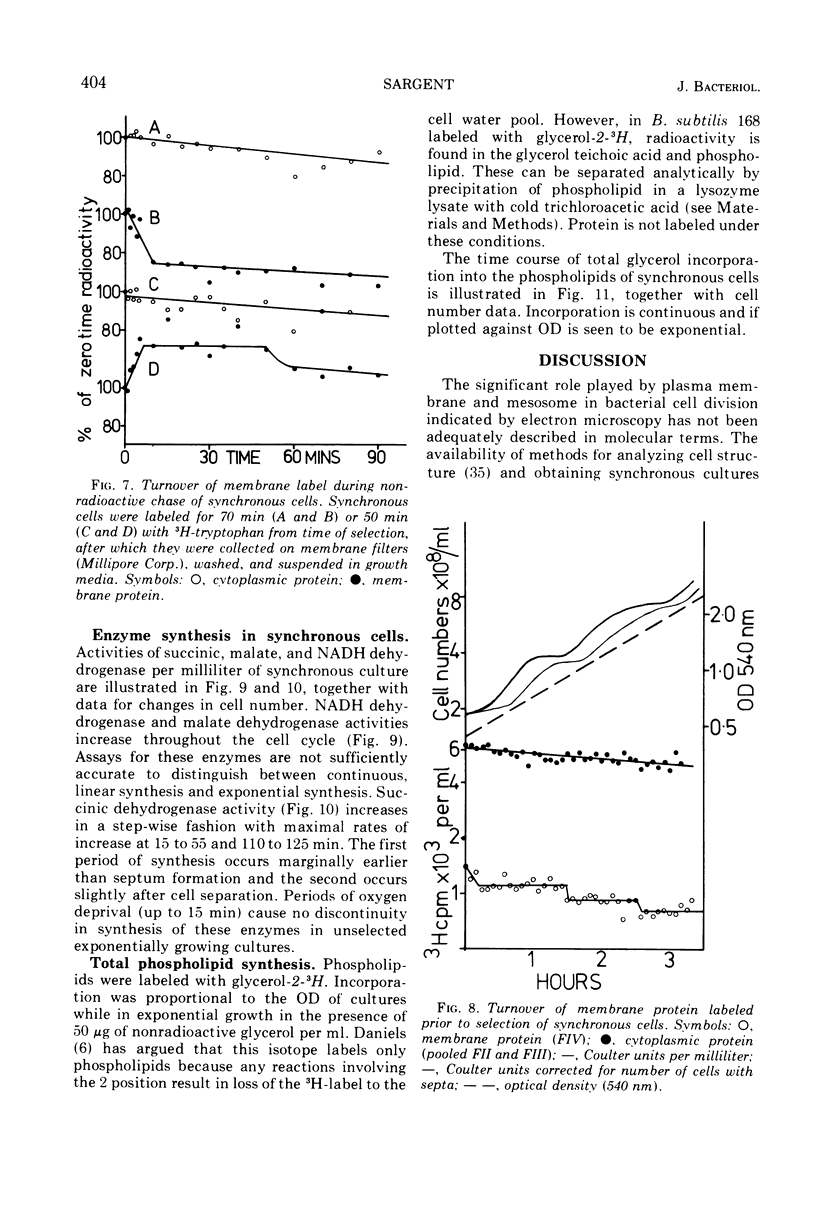
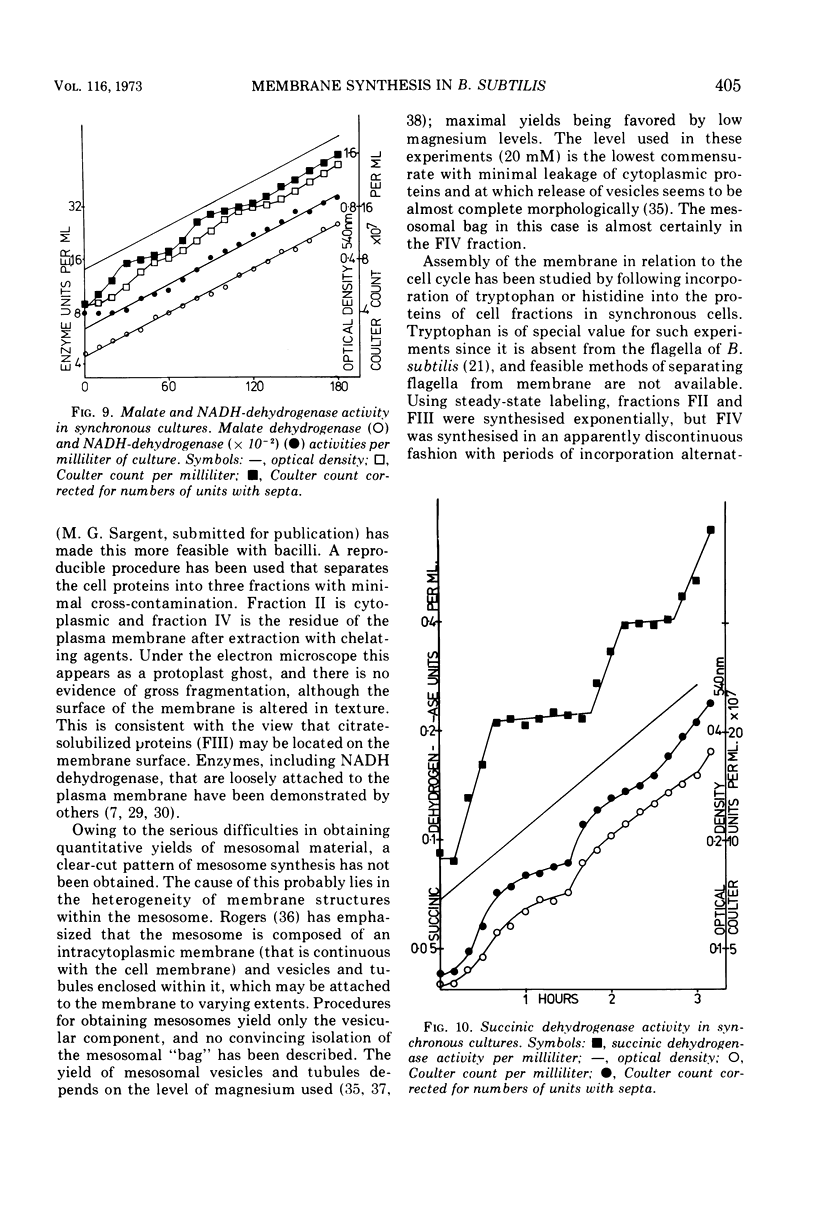
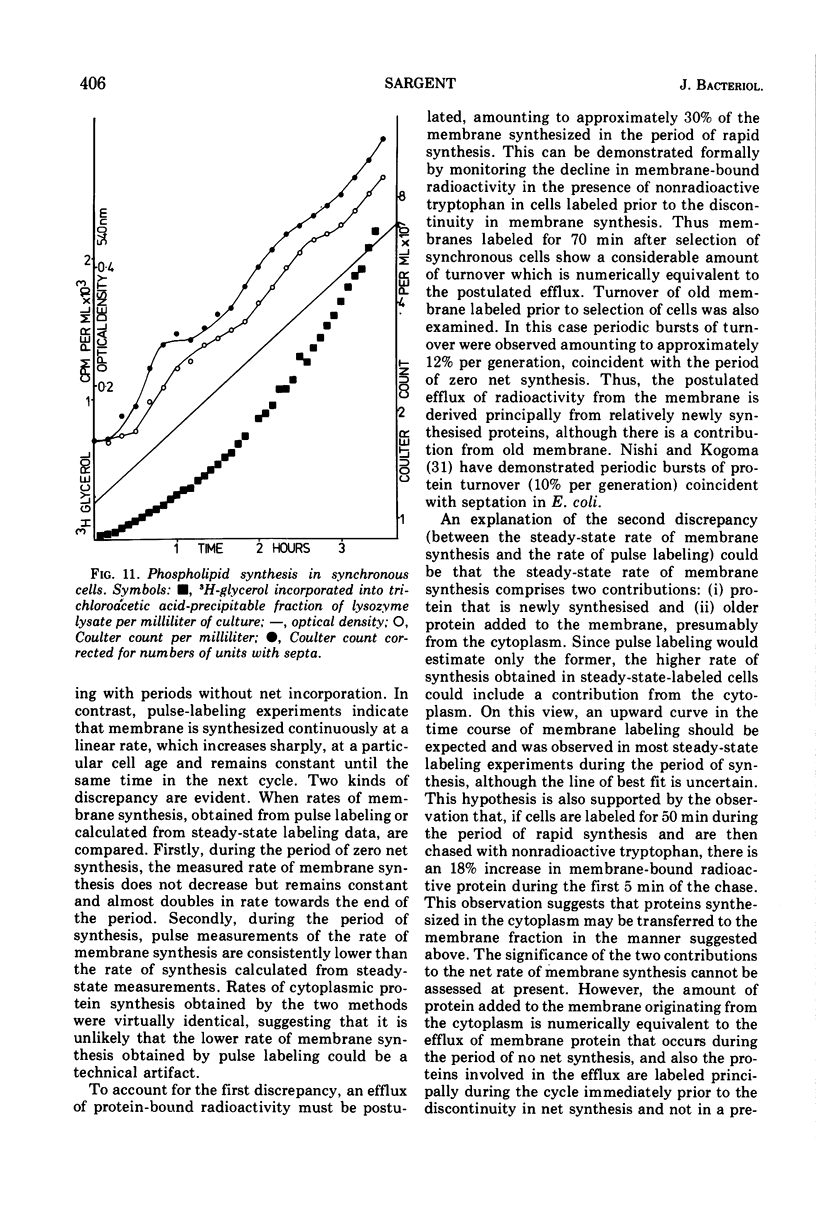
Synthesis of bacterial membranes has been investigated in Bacillus subtilis by examining incorporation of amino acids and glycerol into the protein and lipid of membranes of synchronous cultures. A simple reproducible fractionation scheme divides cellular proteins into three classes (i) truly cytoplasmic, (ii) loosely membrane bound, released by chelating agents, and (iii) tightly membrane bound. These comprise approximately 75, 10, and 15%, respectively, of cellular proteins in this organism. Incorporation of radioactivity into these fractions, using steady-state and pulse labeling has been followed during the cell cycle. Cytoplasmic proteins and the loosely membrane-bound proteins are labeled at an exponential rate throughout the cell cycle. The membrane fraction is labeled discontinuously in the cell cycle, with periods of rapid synthesis over the latter part of the cycle and a period with no net synthesis during the early part of the cycle. Pulse labeling indicates that synthesis of membrane occurs at a linear rate that doubles at a fixed time in each cycle, which coincides with the period of zero net synthesis. Rates of membrane synthesis measured by pulse labeling during the period of rapid membrane synthesis are significantly less than indicated by steady-state labeling. These discrepancies are consistent with the hypothesis that during the cell cycle certain proteins are added to the membrane from the cytoplasm and that during the period of zero net synthesis there is an efflux of proteins from the membrane. Evidence in favor of this has been presented. The activity of succinic dehydrogenase (a representative of class c) varies in a step-wise manner with periods of rapid increase, approximately coincident with bursts of membrane protein synthesis, alternating with periods without any increase in activity. The activities of malate dehydrogenase (class a) and reduced nicotinamide adenine dinucleotide dehydrogenase (class b) increased throughout the cell cycle. Phospholipid synthesis is continuous throughout the cell cycle.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autissier F., Kepes A. Segregation of membrane markers during cell division in Escherichia coli. II. Segregation of Lac-permease and Mel-permease studied with a penicillin technique. Biochim Biophys Acta. 1971 Dec 3;249(2):611–615. doi: 10.1016/0005-2736(71)90141-6. [DOI] [PubMed] [Google Scholar]
- Bellino F. L. Continuous synthesis of partially derepressed aspartate transcarbamylase during the division cycle of Escherichia coli B-r. J Mol Biol. 1973 Feb 25;74(2):223–238. doi: 10.1016/0022-2836(73)90108-3. [DOI] [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Radioautographic evidence for equatorial wall growth in a gram-positive bacterium. Segregation of choline-3H-labeled teichoic acid. J Cell Biol. 1970 Dec;47(3):786–790. doi: 10.1083/jcb.47.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai N. C., Lark K. G. Segregation of deoxyribonucleic acid in bacteria: association of the segregating unit with the cell envelope. J Bacteriol. 1967 Aug;94(2):415–421. doi: 10.1128/jb.94.2.415-421.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. M. Symposium on the fine structure and replication of bacteria and their parts. 3. Bacterial cell-wall replication followed by immunofluorescence. Bacteriol Rev. 1965 Sep;29(3):326–344. doi: 10.1128/br.29.3.326-344.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M. J. Lipid synthesis in relation to the cell cycle of Bacillus megaterium KM and Escherichia coli. Biochem J. 1969 Dec;115(4):697–701. doi: 10.1042/bj1150697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Siervo A. J., Salton M. R. Biosynthesis of cardiolipin in the membranes of Micrococcus lysodeikticus. Biochim Biophys Acta. 1971 Jul 13;239(2):280–292. doi: 10.1016/0005-2760(71)90174-3. [DOI] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J. Growth of the bacterial cell. Nature. 1970 Sep 19;227(5264):1220–1224. doi: 10.1038/2271220a0. [DOI] [PubMed] [Google Scholar]
- Green E. W., Schaechter M. The mode of segregation of the bacterial cell membrane. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2312–2316. doi: 10.1073/pnas.69.8.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter C. E. Origin and sequence of chromosome replication in Escherichia coli B-r. J Bacteriol. 1968 May;95(5):1634–1641. doi: 10.1128/jb.95.5.1634-1641.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B., Messer W., Schwarz U. Regulation of polar cap formation in the life cycle of Escherichia coli. J Supramol Struct. 1972;1(1):29–37. doi: 10.1002/jss.400010105. [DOI] [PubMed] [Google Scholar]
- Hughes R. C., Stokes E. Cell wall growth in Bacillus licheniformis followed by immunofluorescence with mucopeptide-specific antiserum. J Bacteriol. 1971 May;106(2):694–696. doi: 10.1128/jb.106.2.694-696.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahane I., Razin S. Synthesis and turnover of membrane protein and lipid in Mycoplasma laidlawii. Biochim Biophys Acta. 1969 Jun 3;183(1):79–89. doi: 10.1016/0005-2736(69)90131-x. [DOI] [PubMed] [Google Scholar]
- Kennett R. H., Sueoka N. Gene expression during outgrowth of Bacillus subtilis spores. The relationship between gene order on the chromosome and temporal sequence of enzyme synthesis. J Mol Biol. 1971 Aug 28;60(1):31–44. doi: 10.1016/0022-2836(71)90445-1. [DOI] [PubMed] [Google Scholar]
- Kubitschek H. E. Evidence for the generality of linear cell growth. J Theor Biol. 1970 Jul;28(1):15–29. doi: 10.1016/0022-5193(70)90061-5. [DOI] [PubMed] [Google Scholar]
- Kubitschek H. E., Freedman M. L., Silver S. Potassium uptake in synchronous and synchronized cultures of Escherichia coli. Biophys J. 1971 Oct;11(10):787–797. doi: 10.1016/S0006-3495(71)86254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin E. C., Hirota Y., Jacob F. On the process of cellular division in Escherichia coli. VI. Use of a methocel-autoradiographic method for the study of cellular division in Escherichia coli. J Bacteriol. 1971 Oct;108(1):375–385. doi: 10.1128/jb.108.1.375-385.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R. I., Schjeide O. A. Micro estimation of RNA by the cupric ion catalyzed orcinol reaction. Anal Biochem. 1969 Mar;27(3):473–483. doi: 10.1016/0003-2697(69)90061-x. [DOI] [PubMed] [Google Scholar]
- Martinez R. J., Brown D. M., Glazer A. N. The formation of bacterial flagella. 3. Characterization of the subunits of the flagella of Bacillus subtilis and Spirillum serpens. J Mol Biol. 1967 Aug 28;28(1):45–51. doi: 10.1016/s0022-2836(67)80076-7. [DOI] [PubMed] [Google Scholar]
- Masters M., Donachie W. D. Repression and the control of cyclic enzyme synthesis in Bacillus subtilis. Nature. 1966 Jan 29;209(5022):476–479. doi: 10.1038/209476a0. [DOI] [PubMed] [Google Scholar]
- Masters M., Pardee A. B. Sequence of enzyme synthesis and gene replication during the cell cycle of Bacillus subtilis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):64–70. doi: 10.1073/pnas.54.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L., Williamson J. Mode of cell wall growth of Bacillus megaterium. J Bacteriol. 1972 Jan;109(1):373–378. doi: 10.1128/jb.109.1.373-378.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Dales S. Membrane synthesis in Bacillus subtilis. 3. The morphological localization of the sites of membrane synthesis. J Cell Biol. 1972 Oct;55(1):32–41. doi: 10.1083/jcb.55.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. II. Integration of membrane proteins in the absence of lipid synthesis. J Mol Biol. 1970 Apr 28;49(2):433–439. doi: 10.1016/0022-2836(70)90255-x. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Morowitz H. J. Studies on membrane synthesis in Bacillus megaterium KM. J Mol Biol. 1970 Apr 28;49(2):441–459. doi: 10.1016/0022-2836(70)90256-1. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Nachbar M. S., Schor M. T., Salton M. R. Adenosinetriphosphatase of Micrococcus lysodeikticus: selective release and relationship to membrane structure. Biochem Biophys Res Commun. 1968 Aug 13;32(3):539–546. doi: 10.1016/0006-291x(68)90696-7. [DOI] [PubMed] [Google Scholar]
- Nachbar M. S., Salton M. R. Characteristics of a lipid-rich NADH dehydrogenase-containing particulate fraction obtained from Micrococcus lysodeikticus membranes. Biochim Biophys Acta. 1970 Dec 8;223(2):309–320. doi: 10.1016/0005-2728(70)90187-8. [DOI] [PubMed] [Google Scholar]
- Nishi A., Kogoma T. Protein turnover in the cell cycle of Escherichia coli. J Bacteriol. 1965 Oct;90(4):884–890. doi: 10.1128/jb.90.4.884-890.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki M. Correlation between metabolism of phosphatidylglycerol and membrane synthesis in Escherichia coli. J Mol Biol. 1972 Jul 21;68(2):249–264. doi: 10.1016/0022-2836(72)90212-4. [DOI] [PubMed] [Google Scholar]
- Pato M. L., Glaser D. A. The origin and direction of replication of the chromosome of Escherichia coli B-r. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1268–1274. doi: 10.1073/pnas.60.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Heterogeneity of protein turnover in Escherichia coli. Biochim Biophys Acta. 1965 Jul 8;104(2):439–456. doi: 10.1016/0304-4165(65)90349-1. [DOI] [PubMed] [Google Scholar]
- Reaveley D. A., Rogers H. J. Some enzymic activities and chemical properties of the mesosomes and cytoplasmic membranes of Bacillus licheniformis 6346. Biochem J. 1969 Jun;113(1):67–79. doi: 10.1042/bj1130067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Frehel C., Ferrandes B. Comportement des mésosomes lors de l'attaque de Bacillus subtilis par le lysozyme en milieu hyper- ou hypotonique. C R Acad Sci Hebd Seances Acad Sci D. 1967 Oct 23;265(17):1259–1262. [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Steinberg W., Halvorson H. O. Timing of enzyme synthesis during outgrowth of spores of Bacillus cereus. I. Ordered enzyme synthesis. J Bacteriol. 1968 Feb;95(2):469–478. doi: 10.1128/jb.95.2.469-478.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukagoshi N., Fielding P., Fox C. F. Membrane assembly in Escherichia coli. I. Segregation of preformed and newly formed membrane into daughter cells. Biochem Biophys Res Commun. 1971 Jul 16;44(2):497–502. doi: 10.1016/0006-291x(71)90629-2. [DOI] [PubMed] [Google Scholar]
- URBA R. C. Protein breakdown in Bacillus cereus. Biochem J. 1959 Mar;71(3):513–518. doi: 10.1042/bj0710513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vambutas V. K., Salton M. R. Differential inhibitory effects of chloramphenicol on the synthesis of membrane ATPase and cytoplasmic enzymes of Micrococcus lysodeikticus. Biochim Biophys Acta. 1970 Mar 17;203(1):94–103. doi: 10.1016/0005-2736(70)90039-8. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilson G., Fox C. F. Membrane assembly in Escherichia coli. II. Segregation of preformed and newly formed membrane proteins into cells and minicells. Biochem Biophys Res Commun. 1971 Jul 16;44(2):503–509. doi: 10.1016/0006-291x(71)90630-9. [DOI] [PubMed] [Google Scholar]