Abstract
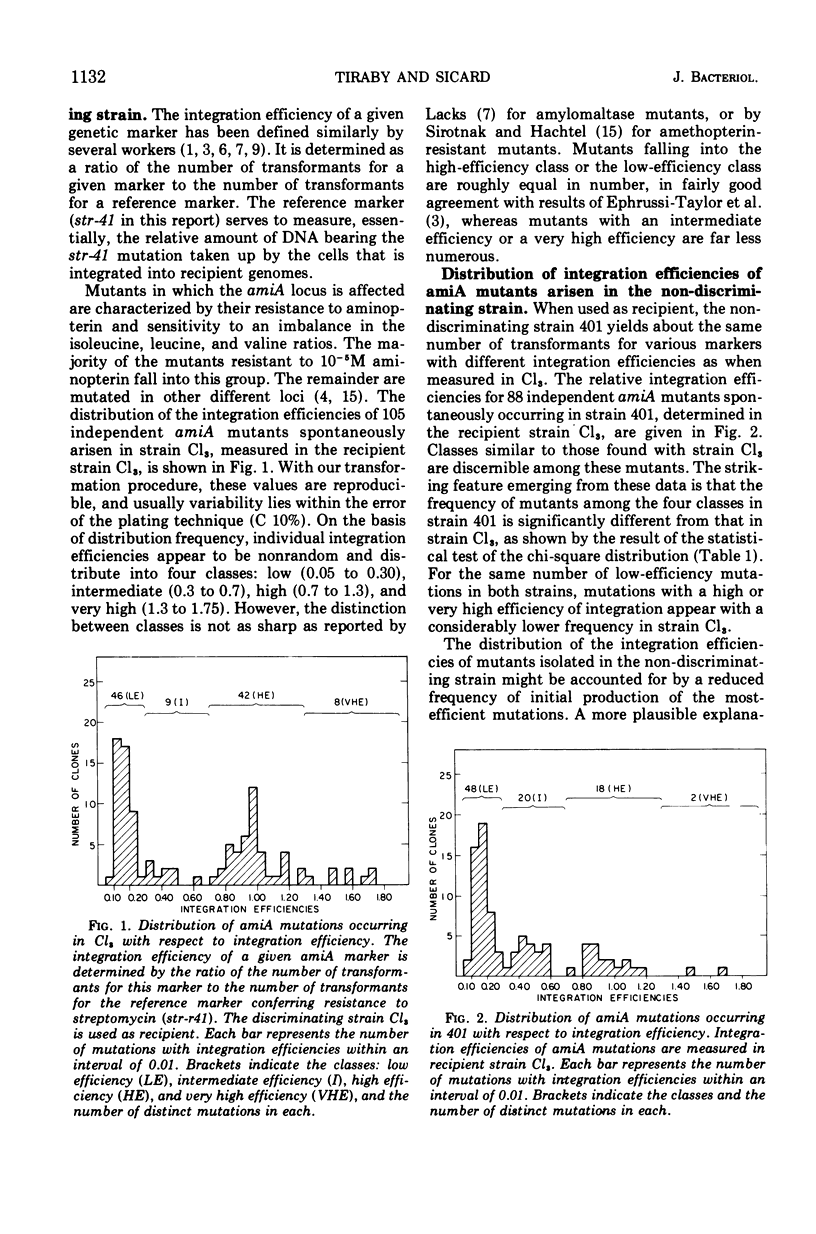
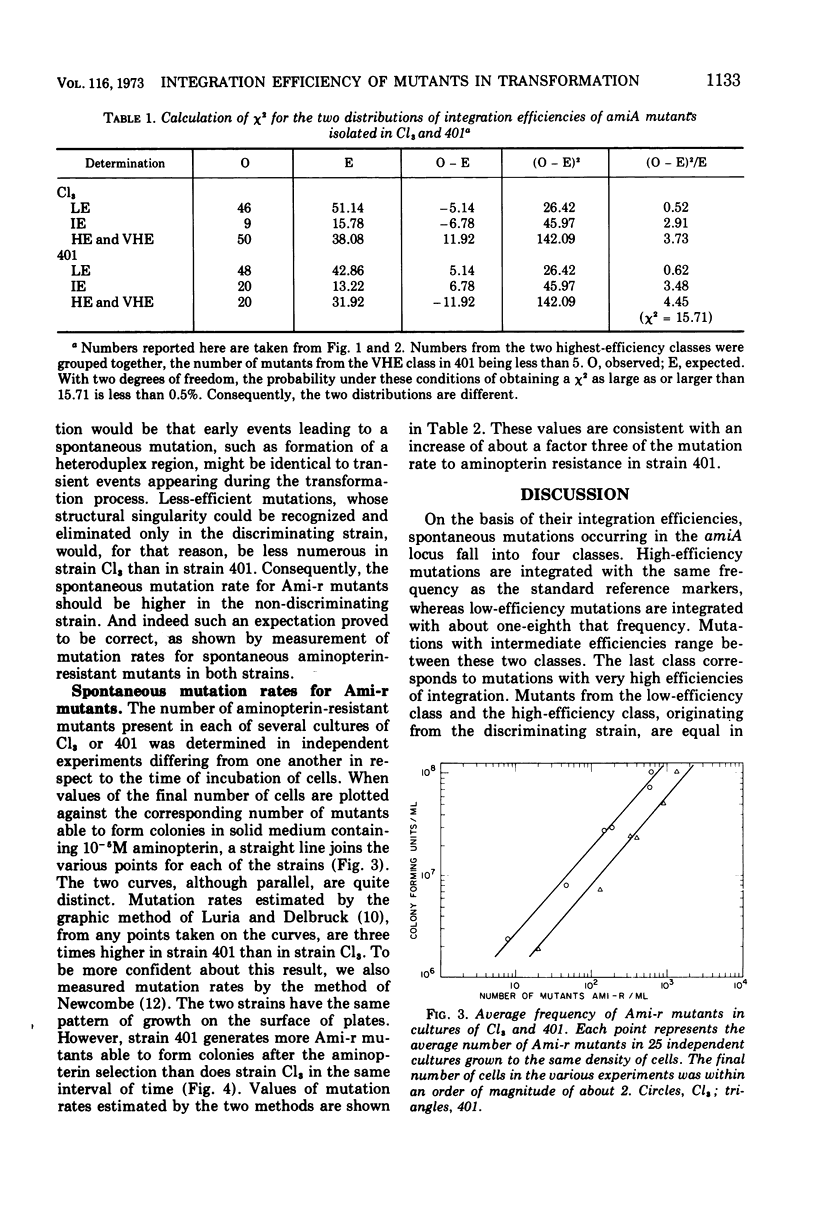
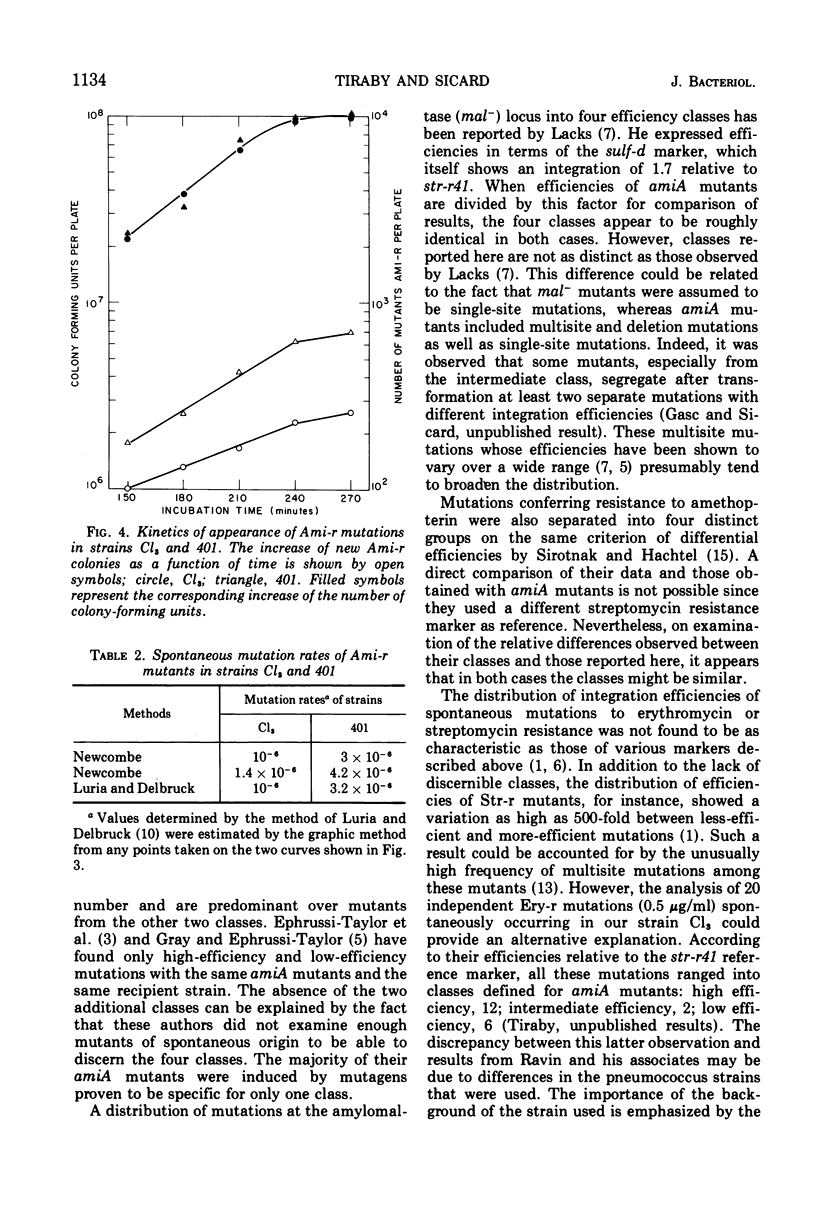
The distribution of integration efficiencies of independent mutations spontaneously occurring in the amiA locus was determined in two strains of pneumococcus. Strain Cl3 integrates genetic markers with different efficiencies during transformation, whereas strain 401, isogenic with strain Cl3, does not discriminate between markers and integrates all markers with the same high efficiency. The discriminating strain Cl3 gives rise to spontaneous mutations in the locus amiA, which fall into four classes with respect to their individual integration efficiencies. Mutations with a low efficiency of integration are equal in number to mutations with a high efficiency. Mutations from the two other classes corresponding to intermediate and very high efficiencies are about five times less frequent. The same four classes were also found among amiA mutants spontaneously occurring in strain 401. However, the two distributions of integration efficiencies of amiA mutants arisen either in strain Cl3 or strain 401 are significantly different. The number of spontaneous amiA mutants, estimated by two methods, was found to be higher in strain 401 than in strain Cl3. The increase of the mutation rate in strain 401 could be accounted for by the excess of mutations falling in the two less-efficient classes observed in this strain. The discriminating process which acts during transformation presumably also intervenes in the appearance of spontaneous mutations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ephrussi-Taylor H., Sicard A. M., Kamen R. Genetic Recombination in DNA-Induced Transformation of Pneumococcus. I. the Problem of Relative Efficiency of Transforming Factors. Genetics. 1965 Mar;51(3):455–475. doi: 10.1093/genetics/51.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L. R., Ravin A. W. Genetic and biochemical properties of thymidine-dependent mutants of pneumococcus. J Bacteriol. 1972 Jan;109(1):459–461. doi: 10.1128/jb.109.1.459-461.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray T. C., Ephrussi-Taylor H. Genetic recombination in DNA-induced transformation of pneumococcus. V. The sbsence of interference, and evidence for the selective elimination of certain donor sites from the final recombinants. Genetics. 1967 Sep;57(1):125–153. doi: 10.1093/genetics/57.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer V N, Ravin A W. Integration and Expression of Different Lengths of DNA during the Transformation of Pneumococcus to Erythromycin Resistance. Genetics. 1962 Oct;47(10):1355–1368. doi: 10.1093/genetics/47.10.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. Integration efficiency and genetic recombination in pneumococcal transformation. Genetics. 1966 Jan;53(1):207–235. doi: 10.1093/genetics/53.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse H. G., Lerman L. S. A genetic analysis by transformation of a group of uracil-requiring mutants of Diplococcus pneumoniae. Genetics. 1969 Jan;61(1):41–60. doi: 10.1093/genetics/61.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHEIM M. B., RAVIN A. W. SITES OF BREAKAGE IN THE DNA MOLECULE AS DETERMINED BY RECOMBINATION ANALYSIS OF STREPTOMYCIN-RESISTANCE MUTATIONS IN PNEUMOCOCCUS. Proc Natl Acad Sci U S A. 1964 Jul;52:30–38. doi: 10.1073/pnas.52.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard A. M., Ephrussi-Taylor H. Genetic recombination in DNA-induced transformation of Pneumococcus. II. Mapping the amiA region. Genetics. 1965 Dec;52(6):1207–1227. doi: 10.1093/genetics/52.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotnak F. M., Hachtel S. L. Increased dihydrofolate reductase synthess in Diplococcus pneumoniae following translatable alteration of the structural gene. I. Genotype derivation and recombinational analyses. Genetics. 1969 Feb;61(2):293–312. doi: 10.1093/genetics/61.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]


