Abstract
The role of tooth wear as a proximate cause of senescence in ruminants has recently been highlighted. There are two competing hypotheses to explain variation in tooth height and wear; the diet-quality hypothesis predicting increased wear in low-quality habitats, and the life-history hypothesis predicting molar height to be related to expected longevity. We compared tooth height and wear from roe deer of known age from two contrasting populations of roe deer (Capreolus capreolus) in France: Trois Fontaines (TF) with good habitat and shorter animal life expectancy and Chizé (CH) with poor habitat and longer animal life expectancy. There was no population difference in tooth wear, leading to rejection of the diet-quality hypothesis. However, despite their smaller body size, initial molar height for animals from CH was larger than for animals from TF. This provides the first evidence that variation in longevity between populations can lead to differences in molar height within a species.
Keywords: longevity, Capreolus capreolus, intraspecific variation, molar, selective browser
1. Introduction
Large mammals go through fairly distinct juvenile, sub-adult and prime-age stages before entering a senescent stage associated with decreased survival and reproductive performance (Gaillard et al. 2000). The role of tooth wear as a proximate cause of senescence in ruminants has recently been highlighted (e.g. Loe et al. 2003; Carranza et al. 2004). In ruminants, teeth are highly specialized and have evolved to withstand the extensive action of abrasion and attrition caused by masticatory degradation of vegetative forage (Lucas 2004). Currently, two competing sets of hypotheses are raised to explain the observed sexual difference in tooth height and wear observed in sexually dimorphic ruminants: (i) those related to variation in diet or habitat quality (Loe et al. 2003) and (ii) those related to life-history differences (Carranza et al. 2004).
The diet-quality hypothesis. Owing to the Jarman–Bell principle (Bell 1971; Jarman 1974; Demment & Van Soest 1985), males may eat a lower quality diet than females, which in turn may result in faster tooth wear rate (Loe et al. 2003). In addition, variation in wear rates between populations of the same species is frequently reported and has been suggested to be due to habitat quality (Loison et al. 2001).
The life-history hypothesis. In sexually dimorphic ruminants, males are the bigger sex with a higher mortality in all life stages and a shorter longevity (Loison et al. 1999; Toïgo & Gaillard 2003). Since teeth are permanent and have fixed dimensions early in life, it has been proposed that their initial size can be taken as a measure of total lifetime ‘repair’, and their wear rate as a measure of current expenditure in performance (Carranza et al. 2004). In support of this life-history hypothesis in highly polygynous species, males ‘living fast and dying young’ have a faster wear rate and a shorter initial molar height than females (Carranza et al. 2004).
So far, these hypotheses, which are not mutually exclusive, have mainly been used to explain variation between the sexes of highly polygynous species. No study has related between-population differences in tooth wear to differences in life-history variables within species nor compared intersexual differences in tooth wear in monomorphic species of ungulates, where intersexual variation in life history and diet are less marked. We here do both. The European roe deer (Capreolus capreolus) is a small-sized cervid (adults weigh approx. 20–30 kg) with low sexual size dimorphism (males are only 10% heavier than females; Andersen et al. 1998). Although an earlier study on roe deer used estimates of molar wear for ageing (Hewison et al. 1999), no analysis was performed to link patterns of tooth wear to sex or life-history theory. We compare tooth height and tooth wear of roe deer in two contrasted populations: the population of the weakly productive forest of Chizé (CH) where deer are quite light (22.9 and 25.1 kg for adult females and males, respectively) and enjoy quite long life (approx. 24% of females living for 12 years or more) versus the population of the highly productive forest of Trois Fontaines (TF) where deer are heavier (24.3 and 26.1 kg for adult females and males, respectively) and with a shorter expected lifespan (only 10% of females living for 12 years or more; Gaillard et al. 1993, 1997, 2003; Pettorelli et al. 2006). The diet-quality hypothesis predicts a faster wear in CH (low-quality habitat) than in TF (high-quality habitat), with no specific pattern for tooth height. The life-history hypothesis predicts initial molar size to be largest in CH, at least after adjusting for possible allometric relationships (i.e. that larger animals have larger teeth), with no specific pattern of wear (or possible slower wear in CH if ‘living slower’). We found that initial molar height was larger (both absolute and relative to body size) in CH where animals had a longer life expectancy. This supports the life-history hypothesis.
2. Material and methods
(a) Study areas and populations
The two study sites are fenced reserves managed by the Office National de la Chasse. CH (46°05′ N, 0°25′ W) is a 2614 ha reserve in western France (Poitou–Charentes) and TF (48°43′ N, 2°61′ W) is a 1360 ha reserve in eastern France (Champagne–Ardennes). Both areas are located inside 5000 ha forests principally composed of oak (Quercus sp.) and beech (Fagus sylvatica), but differ with respect to productivity, climatic conditions and vegetation composition (see Pettorelli et al. (2006) for further details and electronic supplementary material, table 1).
(b) Roe deer data
Both populations have been intensively monitored since 1976 (TF) and 1978 (CH) through annual capture–mark–recapture sessions. Every winter in January to February, roe deer are captured and handled (see Gaillard et al. (2003) for details). Sex, year of birth (only for animals first caught within their first year of life), body mass and hind foot length are recorded for all animals caught. Unmarked animals are either exported to other populations or marked with numbered collars to allow later identification from a distance (see Gaillard et al. (2003) for further information on population estimates and productivity).
Jaws were collected from marked individuals of known age found dead within the study areas (n=93, electronic supplementary material, table 2). Adult body mass, with an accuracy of 500 g, was obtained for most animals during the annual capture–mark–recapture sessions. Mandibular length was measured according to Langvatn (1977) with an accuracy of 1.0 mm. The height of molar 1 was measured with a digital calliper (accuracy of 0.01 mm) as the perpendicular distance from the peak of the distobuccal cusp to the enamel/cementum line (Loe et al. 2003).
(c) Statistical analysis
We used molar height (mm) as the response variable, sex and population (CH versus TF) as factors, and age (years) as a continuous covariate in a linear model. Standard diagnostic tools were applied to assess the fit of the model. We report differences between levels with ‘treatment’ contrasts, i.e. comparing levels of a factor with a reference level. We used the Akaike Information Criterion (AIC; Crawley 2002), corrected for small samples (AICc), to select the most parsimonious model (table 1). To get balanced data between the populations, we restricted the analysis to individuals less than 14 years of age (n=90). All analyses were done in R (R Development Core Team 2004).
Table 1.
Model selection performed on molar height for animals less than 14-years old (n=90). (x, factor included; AIC, Akaike Information Criterion; AICc, AIC corrected for small samples; K, number of parameters. We did not consider more detailed fit to age (e.g. higher order polynomials) due to limited data from older age classes.)
| age | sex | location | age×sex | age×location | age×sex×location | AIC | K | AICc | ΔAICc |
|---|---|---|---|---|---|---|---|---|---|
| x | 254.344 | 2 | 251.477 | 20.619 | |||||
| x | x | 256.086 | 3 | 252.352 | 21.494 | ||||
| x | x | x | 255.850 | 4 | 251.295 | 20.436 | |||
| x | x | 235.992 | 3 | 232.258 | 1.400 | ||||
| x | x | x | 237.672 | 4 | 233.116 | 2.257 | |||
| x | x | x | 237.700 | 4 | 233.145 | 2.286 | |||
| x | x | x | x | 236.192 | 5 | 230.859 | 0.000 | ||
| x | x | x | x | 239.383 | 5 | 234.050 | 3.191 | ||
| x | x | x | x | x | x | 241.115 | 8 | 233.715 | 2.856 |
3. Results
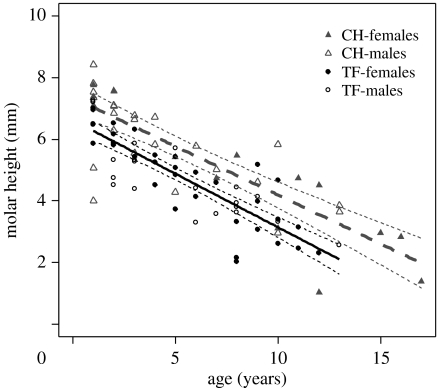
Despite being smaller (electronic supplementary material, figure 1), the longer lived individuals from CH had higher molars than animals from TF (n=90, table 2, figure 1), supporting the life-history hypothesis. Contrary to the prediction from the diet-quality hypothesis, wear rate did not differ between the populations (since the ‘age×population’ interaction term was not included in the best model). However, if nevertheless including ‘age×population (TF versus CH)’ in the model (estimate=0.028 (−0.126, 0.071)), estimated wear was slightly faster in CH. Molar height and wear were similar for males and females as evidenced, respectively, from the main effect of ‘sex’ and the ‘sex×age’ interaction term (table 2). Ln transformation of molar height before analysis yielded similar results.
Table 2.
Parameter estimates of tooth height related to animal age and location for the best model (n=90). (CH, Chizé (reference level for population) and TF, Trois Fontaines.)
| l.s. estimate | s.e. | lower 95% CI | upper 95% CI | |
|---|---|---|---|---|
| intercept | 7.790 | 0.283 | 7.235 | 8.345 |
| age | −0.382 | 0.035 | −0.451 | −0.312 |
| sex | −0.587 | 0.325 | −1.226 | 0.050 |
| location (TF versus CH) | −0.925 | 0.192 | −1.303 | −0.548 |
| age×sex | 0.091 | 0.049 | −0.006 | 0.189 |
Figure 1.
Molar height for roe deer of varying age (indicating wear) from two contrasting populations: Chizé (CH, grey dashed line) and TF (TF, black solid line), France. Note that body size of animals from CH is smaller than from TF (electronic supplementary material, figure 1), while the reverse is true for molar size. Dotted lines are 95% confidence intervals (sexes pooled).
4. Discussion
The most novel finding of this study was that roe deer living in an area with a long life expectancy have larger teeth (figure 1). Roe deer from CH are smaller in size, less productive, with higher juvenile mortality (Gaillard et al. 1997), but with higher adult female survival for old-age classes than for animals inhabiting TF (Gaillard et al. 1993, 1997, 2003). This provides support for the life-history view on tooth height evolution, linking tooth endurance to expected reproductive lifespan (Carranza et al. 2004). The evolution of hypsodonty (i.e. increased molar height) has been linked to increased aridity and thus reduction of forage quality (Fortelius et al. 2002). We failed to find population differences in wear rates, which were expected due to habitat quality. Owing to a fairly limited sample size and since wear may not be linear with age (Loe et al. 2003), it is somewhat premature to reject some role of habitat quality even for the focal populations. The evolutionary processes related to both life history and diet quality, may work in the same direction on molar size, and may well be linked. The longer lifespan expectancy for animals inhabiting an inferior habitat could be explained through stress-responsive survival and its relationship with ‘caloric restriction’ (Sinclair 2005). Caloric restriction has been widely accepted as a cause of extended longevity in numerous species (Masoro 2000; Sinclair 2005). The evolutionary basis of this relies on the presence of a potential future reproductive gain. Since the effects of dental senescence is expected to be most pronounced in mammals living in seasonal habitats (King et al. 2005), our linkage between lifespan and teeth endurance is in correspondence with evolutionary predictions.
Acknowledgments
We acknowledge the financial support of the Research Council of Norway to A.M. (YFF). We are grateful to Leif Egil Loe and two anonymous referees for their constructive comments.
Supplementary Material
References
- Andersen R, Duncan P, Linnell J.D.C. Scandinavian University Press; Oslo, Norway: 1998. The European roe deer: the biology of success. [Google Scholar]
- Bell R.H.V. Grazing ecosystem in Serengeti. Sci. Am. 1971;225:86–93. [Google Scholar]
- Carranza J, Alarcos S, Sanchez-Prieto C.B, Valencia J, Mateos C. Disposable-soma senescence mediated by sexual selection in an ungulate. Nature. 2004;432:215–218. doi: 10.1038/nature03004. doi:10.1038/nature03004 [DOI] [PubMed] [Google Scholar]
- Crawley M.J. Wiley; Chichester, UK: 2002. Statistical computing. An introduction to data analysis using S-Plus. [Google Scholar]
- Demment M.W, Van Soest P.J. A nutritional explanation for body-size patterns of ruminant and nonruminant herbivores. Am. Nat. 1985;125:641–672. doi:10.1086/284369 [Google Scholar]
- Fortelius M, et al. Fossil mammals resolve regional patterns of Eurasian climate change over 20 million years. Evol. Ecol. Res. 2002;4:1005–1016. [Google Scholar]
- Gaillard J.M, Delorme D, Boutin J.M, Van Laere G, Boisaubert B, Pradel R. Roe deer survival patterns—a comparative-analysis of contrasting populations. J. Anim. Ecol. 1993;62:778–791. doi:10.2307/5396 [Google Scholar]
- Gaillard J.M, Boutin J.M, Delorme D, Van Laere G, Duncan P, Lebreton J.D. Early survival in roe deer: causes and consequences of cohort variation in two contrasted populations. Oecologia. 1997;112:502–513. doi: 10.1007/s004420050338. doi:10.1007/s004420050338 [DOI] [PubMed] [Google Scholar]
- Gaillard J.M, Festa-Bianchet M, Yoccoz N.G, Loison A, Toïgo C. Temporal variation in fitness components and population dynamics of large herbivores. Annu. Rev. Ecol. Syst. 2000;31:367–393. doi:10.1146/annurev.ecolsys.31.1.367 [Google Scholar]
- Gaillard J.M, Duncan P, Delorme D, Van Laere G, Pettorelli N, Maillard D, Renaud G. Effects of hurricane Lothar on the population dynamics of European roe deer. J. Wildl. Manage. 2003;67:767–773. [Google Scholar]
- Hewison A.J.M, Vincent J.P, Angibault J.M, Delorme D, Van Laere G, Gaillard J.M. Tests of estimation of age from tooth wear on roe deer of known age: variation within and among populations. Can. J. Zool. 1999;77:58–67. doi:10.1139/cjz-77-1-58 [Google Scholar]
- Jarman P.J. Social-organization of antelope in relation to their ecology. Behaviour. 1974;48:215–267. [Google Scholar]
- King S.J, Arrigo-Nelson S.J, Pochron S.T, Semprebon G.M, Godfrey L.R, Wright P.C, Jernvall J. Dental senescence in a long-lived primate links infant survival to rainfall. Proc. Natl Acad. Sci. USA. 2005;102:16 579–16 583. doi: 10.1073/pnas.0508377102. doi:10.1073/pnas.0508377102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langvatn R. Nordic Council for Wildlife Research; Stockholm, Sweden: 1977. Criteria of physical condition, growth and development in Cervidae, suitable for routine studies. [Google Scholar]
- Loe L.E, Mysterud A, Langvatn R, Stenseth N.C. Decelerating and sex-dependent tooth wear in Norwegian red deer. Oecologia. 2003;135:346–353. doi: 10.1007/s00442-003-1192-9. [DOI] [PubMed] [Google Scholar]
- Loison A, Festa-Bianchet M, Gaillard J.M, Jorgenson J.T, Jullien J.M. Age-specific survival in five populations of ungulates: evidence of senescence. Ecology. 1999;80:2539–2554. doi:10.2307/177239 [Google Scholar]
- Loison A, Cuyler L.C, Linnell J.D.C, Landa A. Sex, age, condition and tooth wear of harvested caribou Rangifer tarandus groenlandicus in west Greenland, 1995–1998. Wildl. Biol. 2001;7:263–273. [Google Scholar]
- Lucas P.W. Cambridge University Press; Cambridge, UK: 2004. Dental functional morphology. [Google Scholar]
- Masoro E.J. Caloric restriction and aging: an update. Exp. Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. doi:10.1016/S0531-5565(00)00084-X [DOI] [PubMed] [Google Scholar]
- Pettorelli N, Gaillard J.M, Mysterud A, Duncan P, Stenseth N.C, Delorme D, Van Laere G, Toigo C, Klein F. Using a proxy of plant productivity (NDVI) to find key periods for animal performance: the case of roe deer. Oikos. 2006;112:565–572. doi:10.1111/j.0030-1299.2006.14447.x [Google Scholar]
- R Development Core Team, 2004 R: A language and environment for statistical computing Vienna, Austria.
- Sinclair D.A. Toward a unified theory of caloric restriction and longevity regulation. Mech. Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. doi:10.1016/j.mad.2005.03.019 [DOI] [PubMed] [Google Scholar]
- Toïgo C, Gaillard J.M. Causes of sex-biased adult survival in ungulates: sexual size dimorphism, mating tactic or environment harshness? Oikos. 2003;101:376–384. doi:10.1034/j.1600-0706.2003.12073.x [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.