Abstract
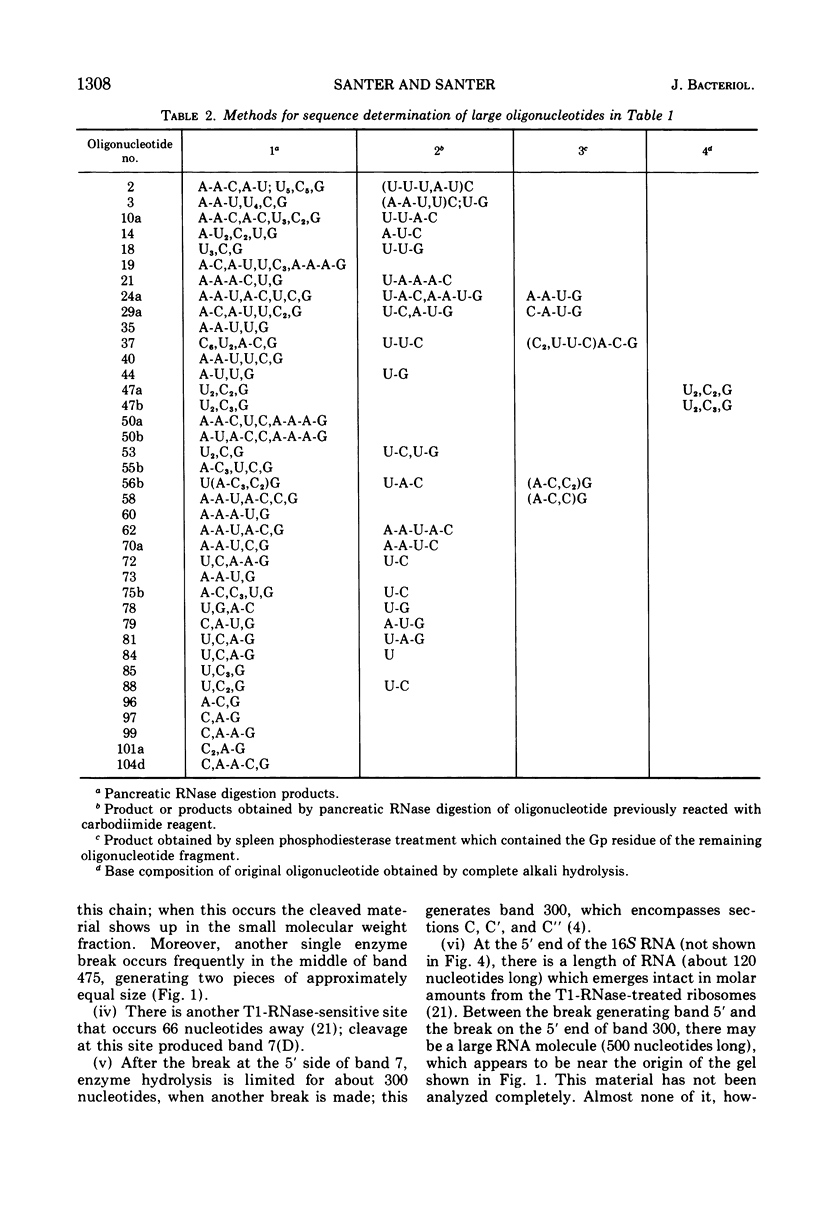
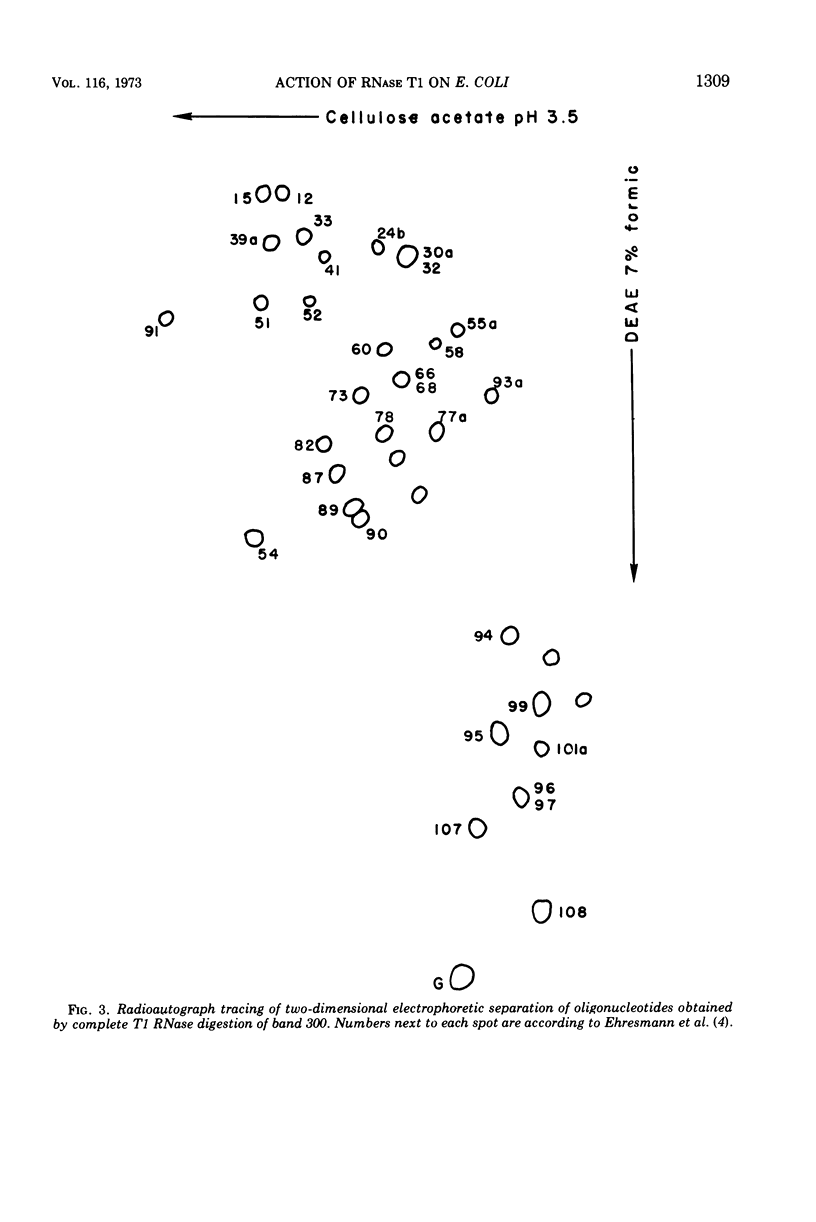
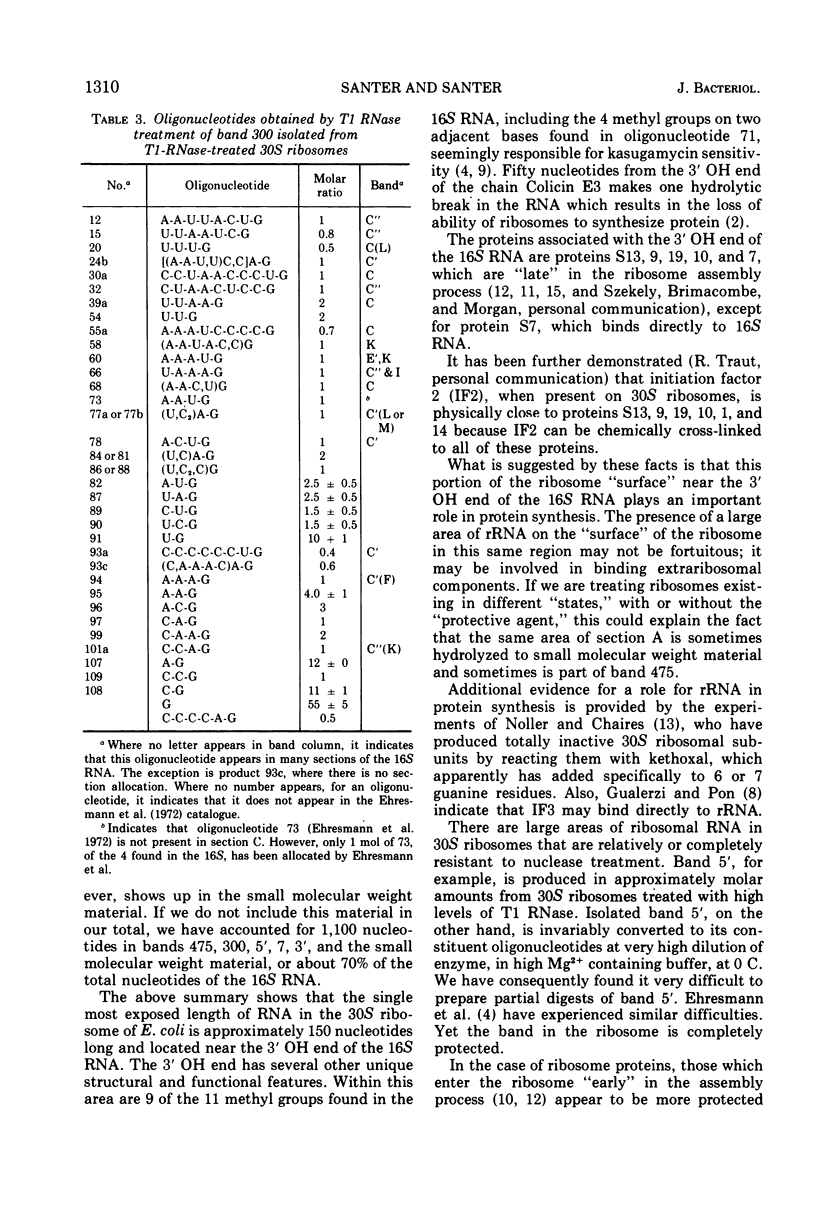
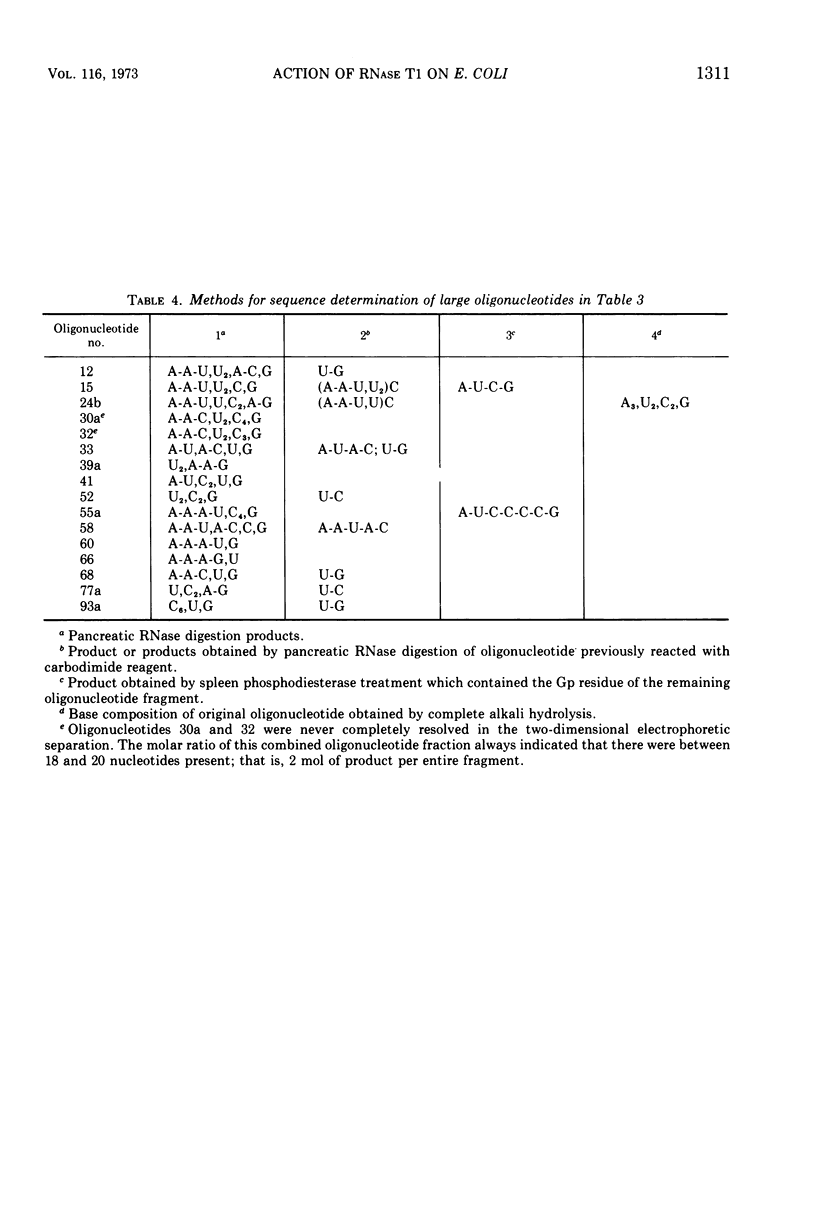
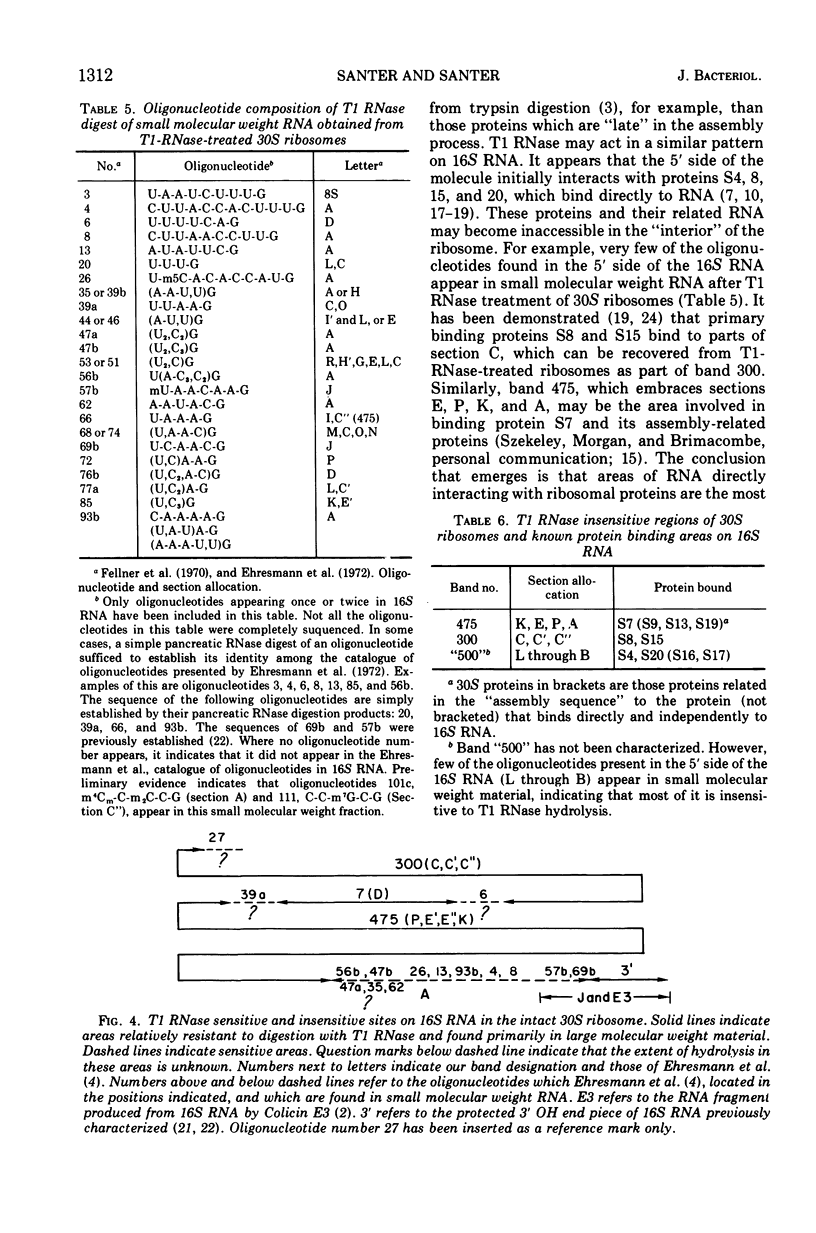
Two large ribonucleic acid (RNA) fragments have been obtained from T1-RNase-treated 30S ribosomes of Escherichia coli. One fragment, about 475 nucleotides long, contains all the unique oligonucleotides found by Fellner and associates in sections of 16S RNA designated P, E, E′, and K, and one-half the large oligonucleotides of section A. The other large fragment is about 300 nucleotides long and contains the oligonucleotides found in sections C, C′, C″. The isolation of these large fragments seems to confirm the arrangement of sections within 16S RNA. There are also recovered from nuclease-treated ribosomes three small fragments, one (120 nucleotides long) from the 5′ end, one (26 nucleotides long) from the 3′ OH end of the chain, and another section (66 nucleotides long) from the middle of the 16S RNA chain. Small molecular weight material is also generated by nuclease treatment, and about half this material is derived from a region close to the 3′ OH end of the 16S RNA chain. This indicates that the most accessible part of the rRNA of E. coli 30S ribosomes is a region 100 to 150 nucleotides long near the 3′ end of the chain. A general scheme is proposed to explain the generation of the various-sized RNA products from the rRNA of the 30S ribosome.
Full text
PDF
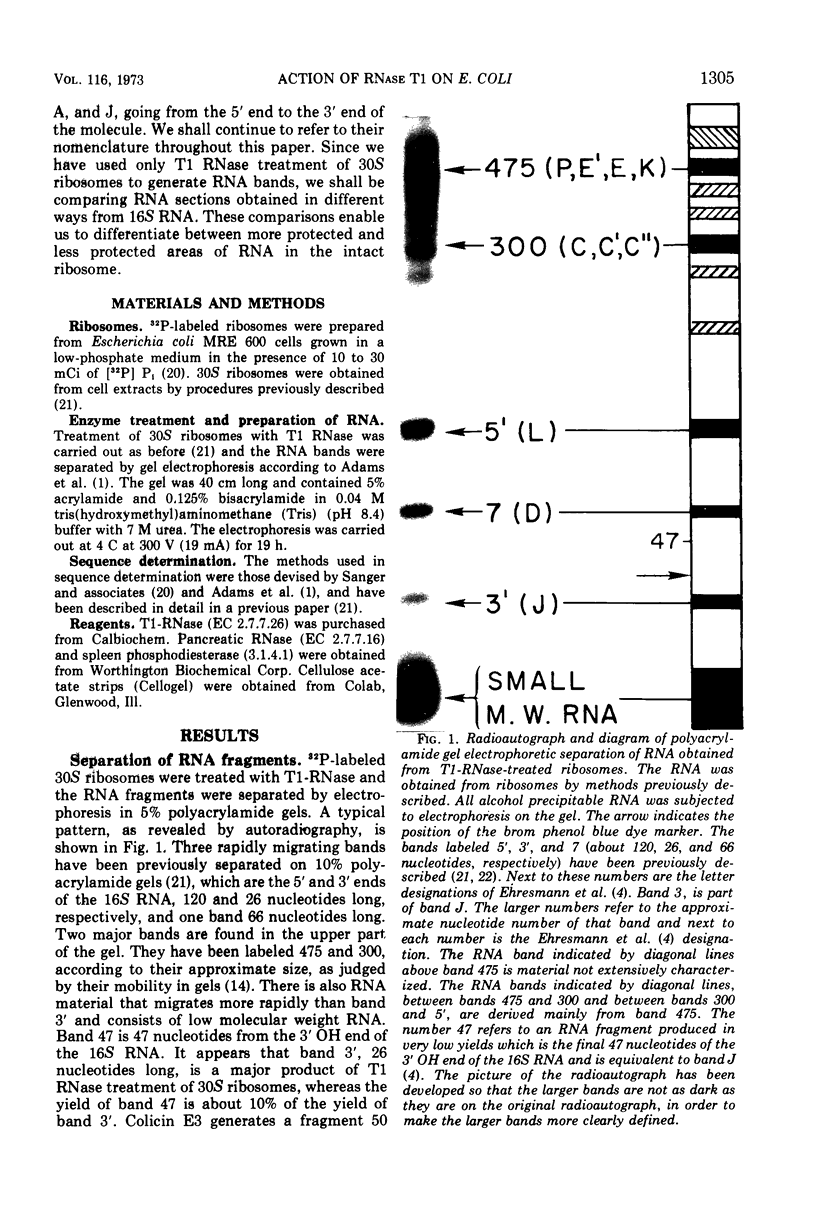

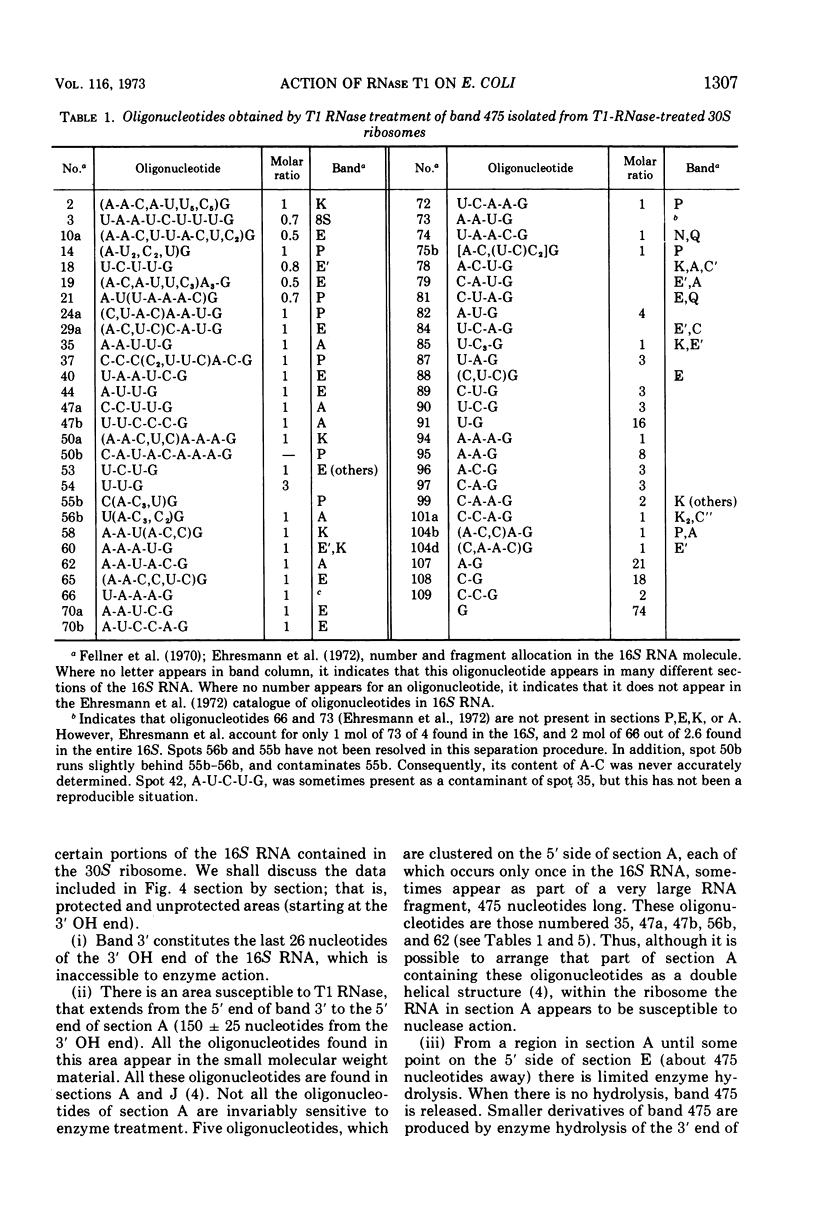

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Jeppesen P. G., Sanger F., Barrell B. G. Nucleotide sequence from the coat protein cistron of R17 bacteriophage RNA. Nature. 1969 Sep 6;223(5210):1009–1014. doi: 10.1038/2231009a0. [DOI] [PubMed] [Google Scholar]
- Bowman C. M., Dahlberg J. E., Ikemura T., Konisky J., Nomura M. Specific inactivation of 16S ribosomal RNA induced by colicin E3 in vivo. Proc Natl Acad Sci U S A. 1971 May;68(5):964–968. doi: 10.1073/pnas.68.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton R. R., Wittmann H. G. Ribosomal proteins. XXIV. Trypsin digestion as a possible probe of the conformation of Escherichia coli ribosomes. Mol Gen Genet. 1972;114(2):95–105. doi: 10.1007/BF00332780. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Fellner P., Ebel J. P. The determination of the primary structure of the 16S ribosomal RNA of Escherichia coli. 2. Nucleotide sequences of products from partial enzymatic hydrolysis. Biochimie. 1972;54(7):901–967. doi: 10.1016/s0300-9084(72)80007-5. [DOI] [PubMed] [Google Scholar]
- Fellner P., Ehresmann C., Ebel J. P. Nucleotide sequences in a protected area of the 16S RNA within 30S ribosomal subunits from Escherichia coli. Eur J Biochem. 1970 Apr;13(3):583–588. doi: 10.1111/j.1432-1033.1970.tb00964.x. [DOI] [PubMed] [Google Scholar]
- Fellner P., Ehresmann C., Ebel J. P. The determination of the primary structure of the 16S ribosomal RNA of Escherichia coli. 1. Nucleotide sequence analysis of T 1 and pancreatic ribonuclease digestion products. Biochimie. 1972;54(7):853–900. doi: 10.1016/s0300-9084(72)80006-3. [DOI] [PubMed] [Google Scholar]
- Garrett R. A., Rak K. H., Daya L., Stöffler G. Ribosomal proteins. XXIX. Specific protein binding sites on 16S rRNA of Escherichia coli. Mol Gen Genet. 1972;114(2):112–124. doi: 10.1007/BF00332782. [DOI] [PubMed] [Google Scholar]
- Gualerzi C., Pon C. L. Nature of the ribosomal binding site for initiation factor 3 (IF-3). Biochem Biophys Res Commun. 1973 Jun 8;52(3):792–799. doi: 10.1016/0006-291x(73)91007-3. [DOI] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Mechanism of kasugamycin resistance in Escherichia coli. Nat New Biol. 1972 Jan 5;235(53):6–9. doi: 10.1038/newbio235006a0. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Nomura M. Assembly mapping of 30S ribosomal proteins from E. coli. Nature. 1970 Jun 27;226(5252):1214–1214. doi: 10.1038/2261214a0. [DOI] [PubMed] [Google Scholar]
- Morgan J., Brimacombe R. A series of specific ribonucleoprotein fragments from the 30-S subparticle of Escherichia coli ribosomes. Eur J Biochem. 1972 Sep 25;29(3):542–552. doi: 10.1111/j.1432-1033.1972.tb02020.x. [DOI] [PubMed] [Google Scholar]
- Nashimoto H., Held W., Kaltschmidt E., Nomura M. Structure and function of bacterial ribosomes. XII. Accumulation of 21 s particles by some cold-sensitive mutants of Escherichia coli. J Mol Biol. 1971 Nov 28;62(1):121–138. doi: 10.1016/0022-2836(71)90135-5. [DOI] [PubMed] [Google Scholar]
- Noller H. F., Chaires J. B. Functional modification of 16S ribosomal RNA by kethoxal. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3115–3118. doi: 10.1073/pnas.69.11.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Roth H. E., Nierhaus K. H. Isolation of four ribonucleoprotein fragments from the 30 S subunit of E. coli ribosomes. FEBS Lett. 1973 Apr 1;31(1):35–38. doi: 10.1016/0014-5793(73)80068-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Santer M., Santer U. V. Characterization of the 5' and 3' ends of the 16S ribonucleic acid from T 1 -ribonuclease treated 30S ribosomes. Biochemistry. 1972 Feb 29;11(5):786–791. doi: 10.1021/bi00755a017. [DOI] [PubMed] [Google Scholar]
- Santer M., Székely M. Nuclease action on Escherichia coli ribosomes and its application to sequence studies on ribosomal ribonucleic acid. Biochemistry. 1971 May 11;10(10):1841–1846. doi: 10.1021/bi00786a018. [DOI] [PubMed] [Google Scholar]
- Santer U. V., Santer M. The sequence of the 3'-OH end of the 16 S RNA of Escherichia coli. FEBS Lett. 1972 Apr 1;21(3):311–314. doi: 10.1016/0014-5793(72)80191-1. [DOI] [PubMed] [Google Scholar]
- Schaup H. W., Green M., Kurland C. G. Molecular interactions of ribosomal components. I. Identification of RNA binding sites for individual 30S ribosomal proteins. Mol Gen Genet. 1970;109(3):193–205. doi: 10.1007/BF00267007. [DOI] [PubMed] [Google Scholar]
- Schaup H. W., Green M., Kurland C. G. Molecular interactions of ribosomal components. II. Site-specific complex formation between 30S proteins and ribosomal RNA. Mol Gen Genet. 1971;112(1):1–8. doi: 10.1007/BF00266926. [DOI] [PubMed] [Google Scholar]
- Schaup H. W., Sogin M., Woese C. Characterization of an RNA "binding site" for a specific ribosomal protein of Escherichia coli. Mol Gen Genet. 1972;114(1):1–8. doi: 10.1007/BF00268740. [DOI] [PubMed] [Google Scholar]
- Zimmermann R. A., Muto A., Fellner P., Ehresmann C., Branlant C. Location of ribosomal protein binding sites on 16S ribosomal RNA. Proc Natl Acad Sci U S A. 1972 May;69(5):1282–1286. doi: 10.1073/pnas.69.5.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]