Abstract
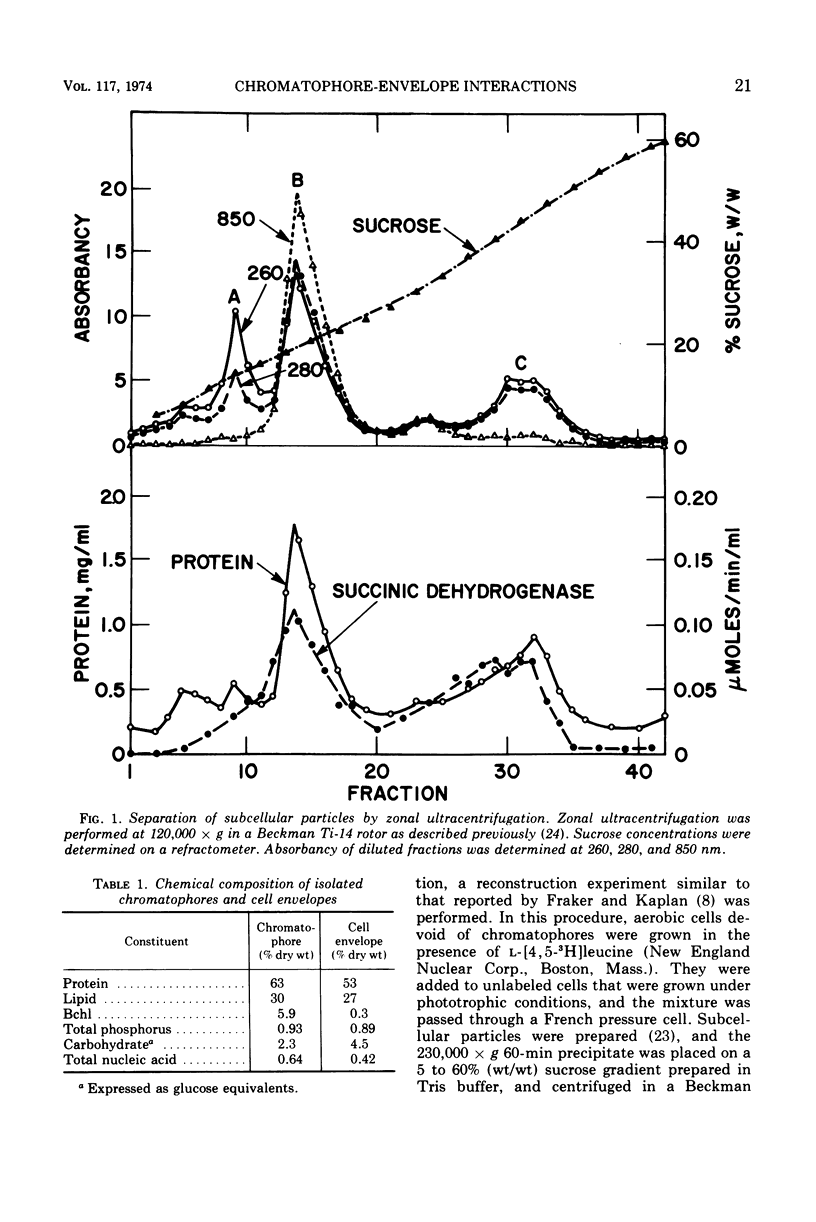
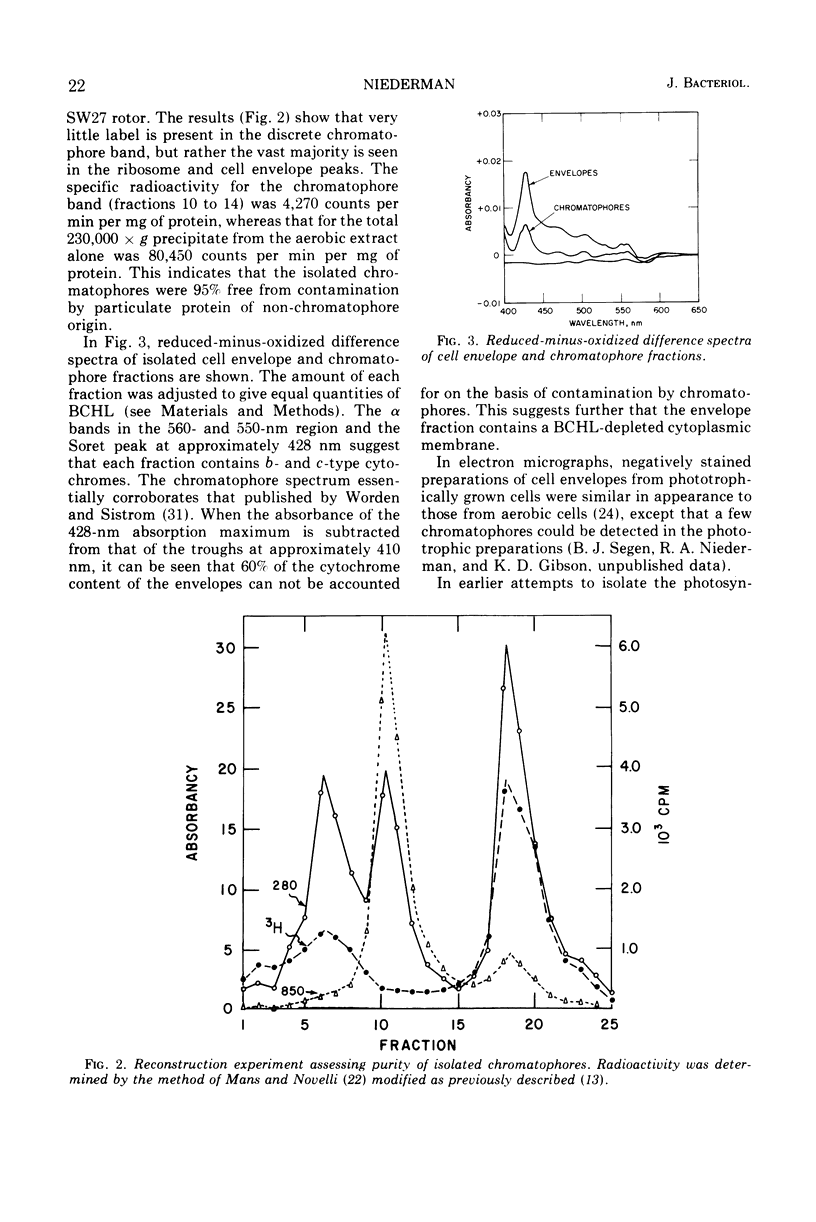
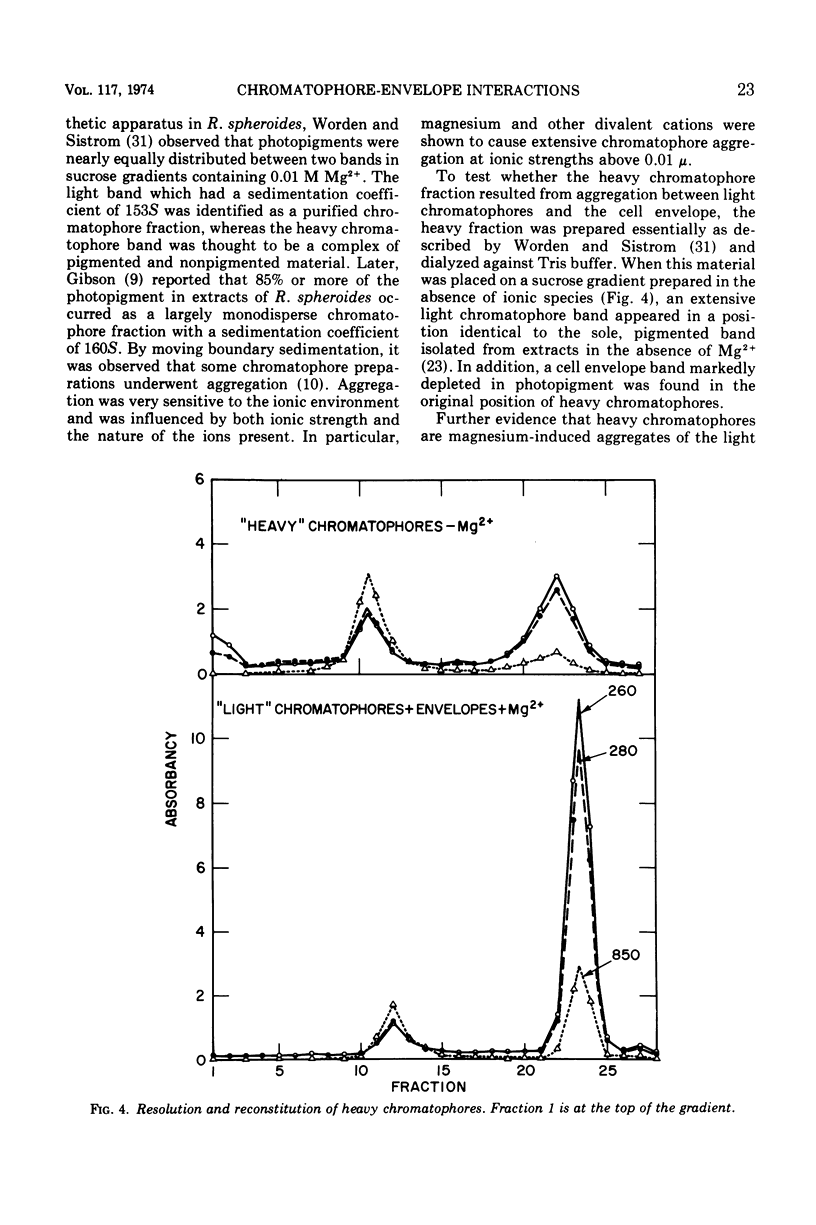
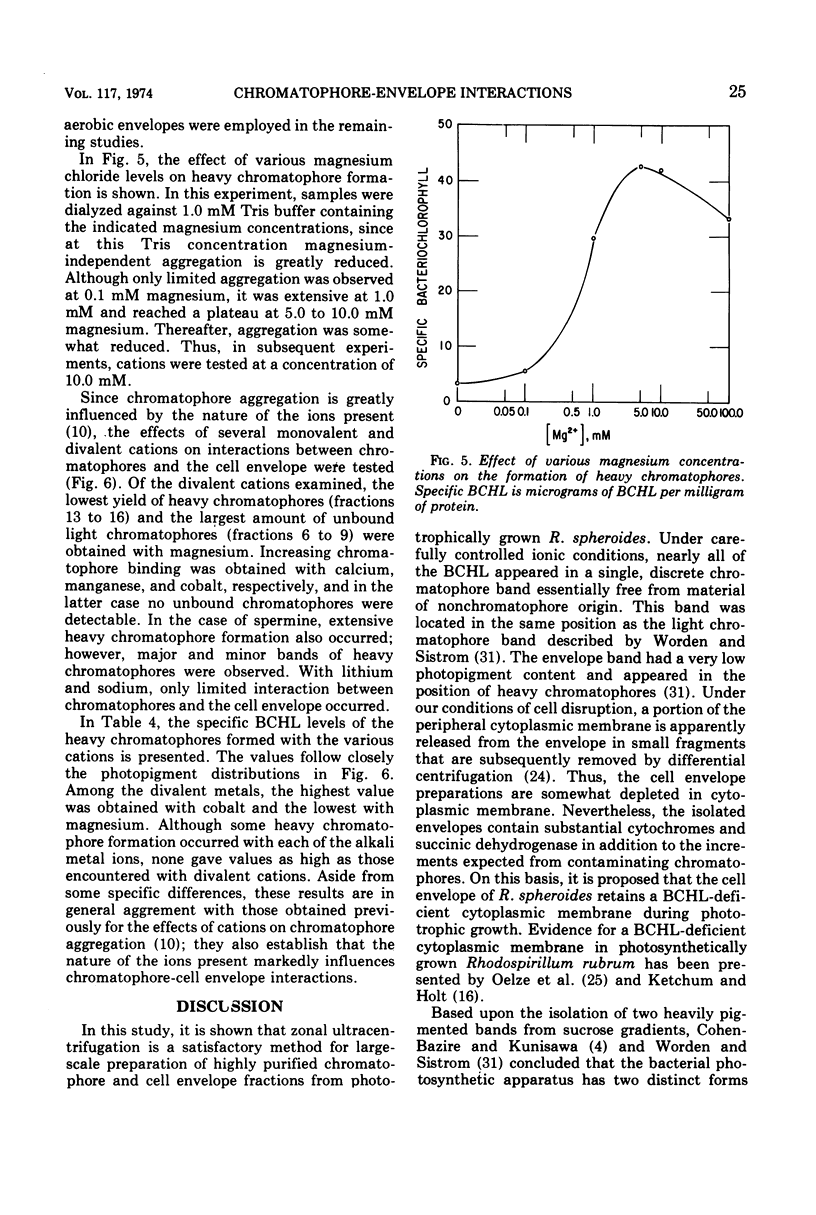
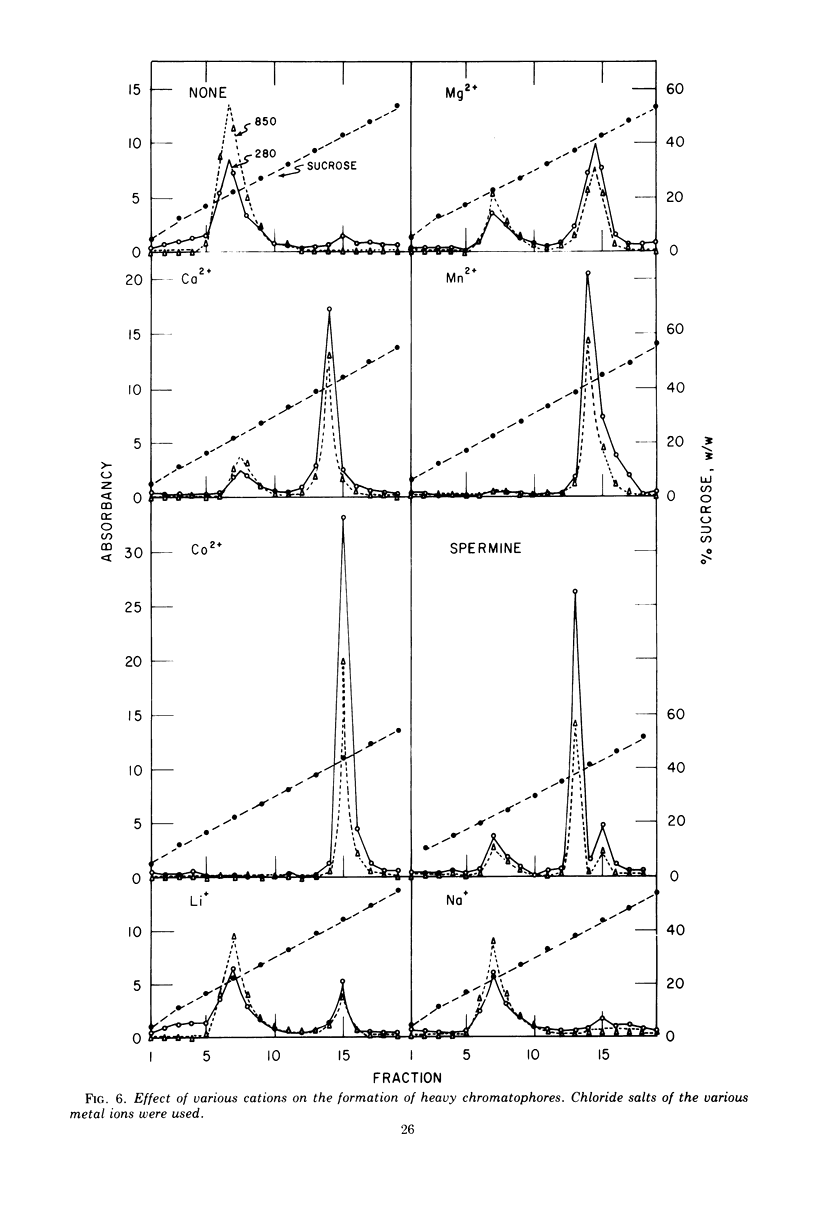
Under carefully controlled ionic conditions, large-scale preparations of highly purified chromatophores and cell envelopes were obtained from phototrophically grown Rhodopseudomonas spheroides by zonal ultracentrifugation. The majority of the bacteriochlorophyll a was located in a single, discrete chromatophore band, whereas the envelopes were nearly devoid of photopigment. The envelope fraction contained substantial quantities of succinic dehydrogenase and cytochromes, confirming that phototrophically grown cells contain a photopigment-deficient cytoplasmic membrane. Magnesium at concentrations of 1.0 mM or higher caused chromatophores to reversibly aggregate with the cell envelope. Significant aggregation was also promoted by other divalent metals (Co2+ > Mn2+ > Ca2+ > Mg2+), but aggregation was less extensive with monovalent cations. These results account for the distribution of photopigments in two bands reported by others and further suggest that the photosynthetic apparatus of R. spheroides is located on membranes largely distinct from the cell wall-cytoplasmic membrane complex.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLAYTON R. K. TOWARD THE ISOLATION OF A PHOTOCHEMICAL REACTION CENTER IN RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1963 Nov 29;75:312–323. doi: 10.1016/0006-3002(63)90618-8. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fraker P. J., Kaplan S. Isolation and fractionation of the photosynthetic membranous organelles from Rhodopseudomonas spheroides. J Bacteriol. 1971 Oct;108(1):465–473. doi: 10.1128/jb.108.1.465-473.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson K. D., Segen B. J., Niederman R. A. Membranes of Rhodopseudomonas spheroides. II. Precursor-product relations in anaerobically growing cells. Arch Biochem Biophys. 1972 Oct;152(2):561–568. doi: 10.1016/0003-9861(72)90251-2. [DOI] [PubMed] [Google Scholar]
- Gorchein A. The separation and identification of the lipids of Rhodopseudomonas spheroides. Proc R Soc Lond B Biol Sci. 1968 Jul 2;170(1020):279–297. doi: 10.1098/rspb.1968.0039. [DOI] [PubMed] [Google Scholar]
- Kahane I., Ne'eman Z., Razin S. Divalent cations in native and reaggregated mycoplasma membranes. J Bacteriol. 1973 Feb;113(2):666–671. doi: 10.1128/jb.113.2.666-671.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchum P. A., Holt S. C. Isolation and characterization of the membranes from Rhodospirillum rubrum. Biochim Biophys Acta. 1970;196(2):141–161. doi: 10.1016/0005-2736(70)90002-7. [DOI] [PubMed] [Google Scholar]
- LASCELLES J., SZILAGYI J. F. PHOSPHOLIPID SYNTHESIS BY RHODOPSEUDOMONAS SPHEROIDES IN RELATION TO THE FORMATION OF PHOTOSYNTHETIC PIGMENTS. J Gen Microbiol. 1965 Jan;38:55–64. doi: 10.1099/00221287-38-1-55. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Niederman R. A., Gibson K. D. The separation of chromatophores from the cell envelope in Rhodopseudomonas spheroides. Prep Biochem. 1971;1(2):141–150. doi: 10.1080/00327487108081935. [DOI] [PubMed] [Google Scholar]
- Niederman R. A., Segen B. J., Gibson K. D. Membranes of Rhodopseudomonas spheroides. I. Isolation and characterization of membrane fractions from extracts of aerobically and anaerobically grown cells. Arch Biochem Biophys. 1972 Oct;152(2):547–560. doi: 10.1016/0003-9861(72)90250-0. [DOI] [PubMed] [Google Scholar]
- Razin S. Reconstruction of biological membranes. Biochim Biophys Acta. 1972 Apr 18;265(2):241–296. [PubMed] [Google Scholar]
- Rottem S., Stein O., Razin S. Reassembly of Mycoplasma membranes disaggregated by detergents. Arch Biochem Biophys. 1968 Apr;125(1):46–56. doi: 10.1016/0003-9861(68)90637-1. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. Observations on the relationship between the formation of photopigments and the synthesis of protein in Rhodopseudomonas spheroides. J Gen Microbiol. 1962 Sep;28:599–605. doi: 10.1099/00221287-28-4-599. [DOI] [PubMed] [Google Scholar]
- Takacs B. J., Holt S. C. Thiocapsa floridana; a cytological, physical and chemical characterization. II. Physical and chemical characteristics of isolated and reconstituted chromatophores. Biochim Biophys Acta. 1971 Apr 13;233(2):278–295. doi: 10.1016/0005-2736(71)90326-9. [DOI] [PubMed] [Google Scholar]
- WOODY B. R., LINDSTROM E. S. The succinic dehydrogenase from Rhodospirillum rubrum. J Bacteriol. 1955 Mar;69(3):353–356. doi: 10.1128/jb.69.3.353-356.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORDEN P. B., SISTROM W. R. THE PREPARATION AND PROPERTIES OF BACTERIAL CHROMATOPHORE FRACTIONS. J Cell Biol. 1964 Oct;23:135–150. doi: 10.1083/jcb.23.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]


