Abstract
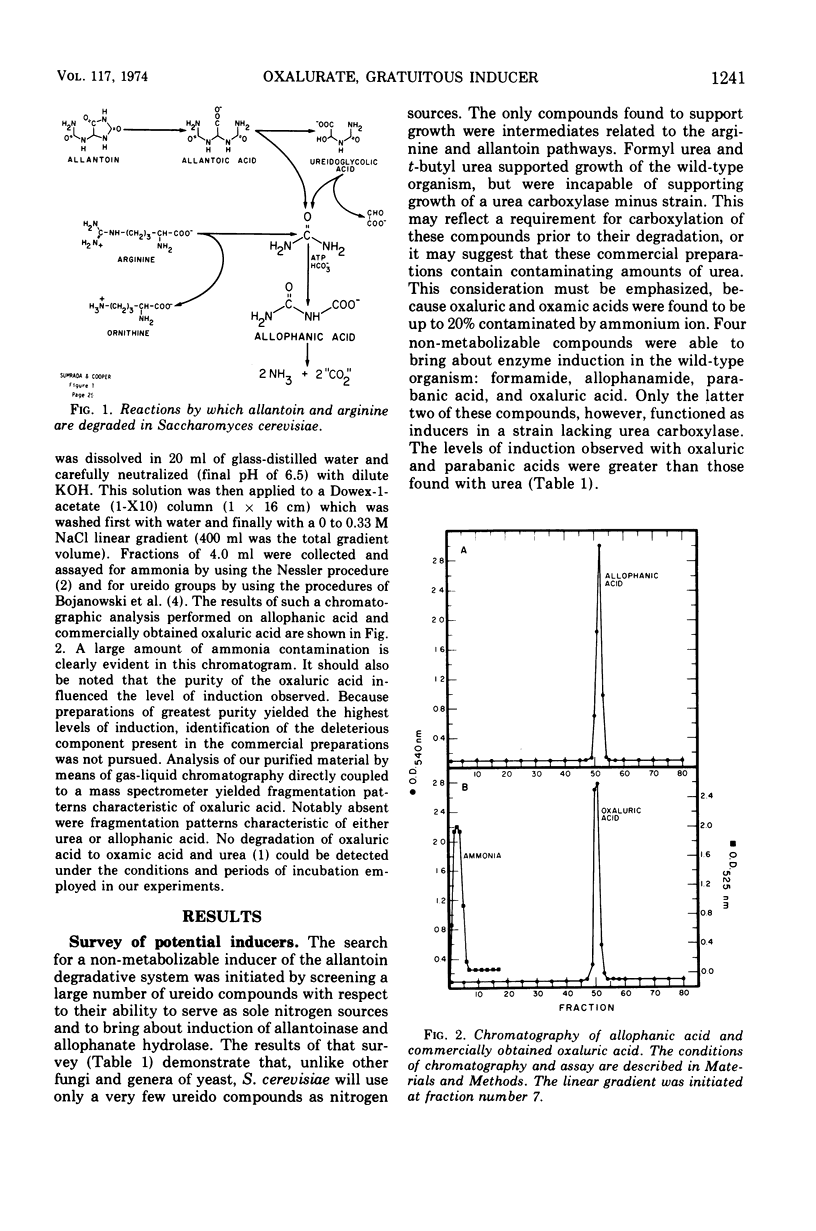
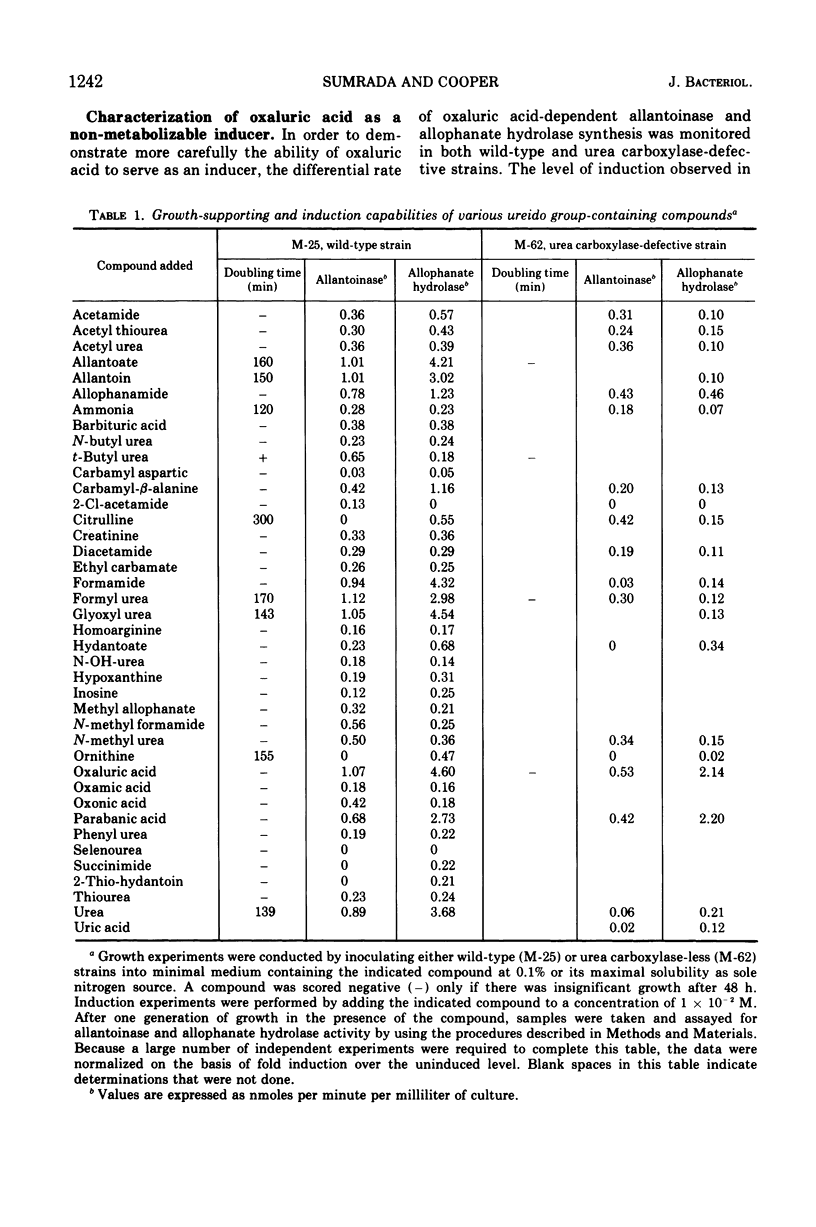
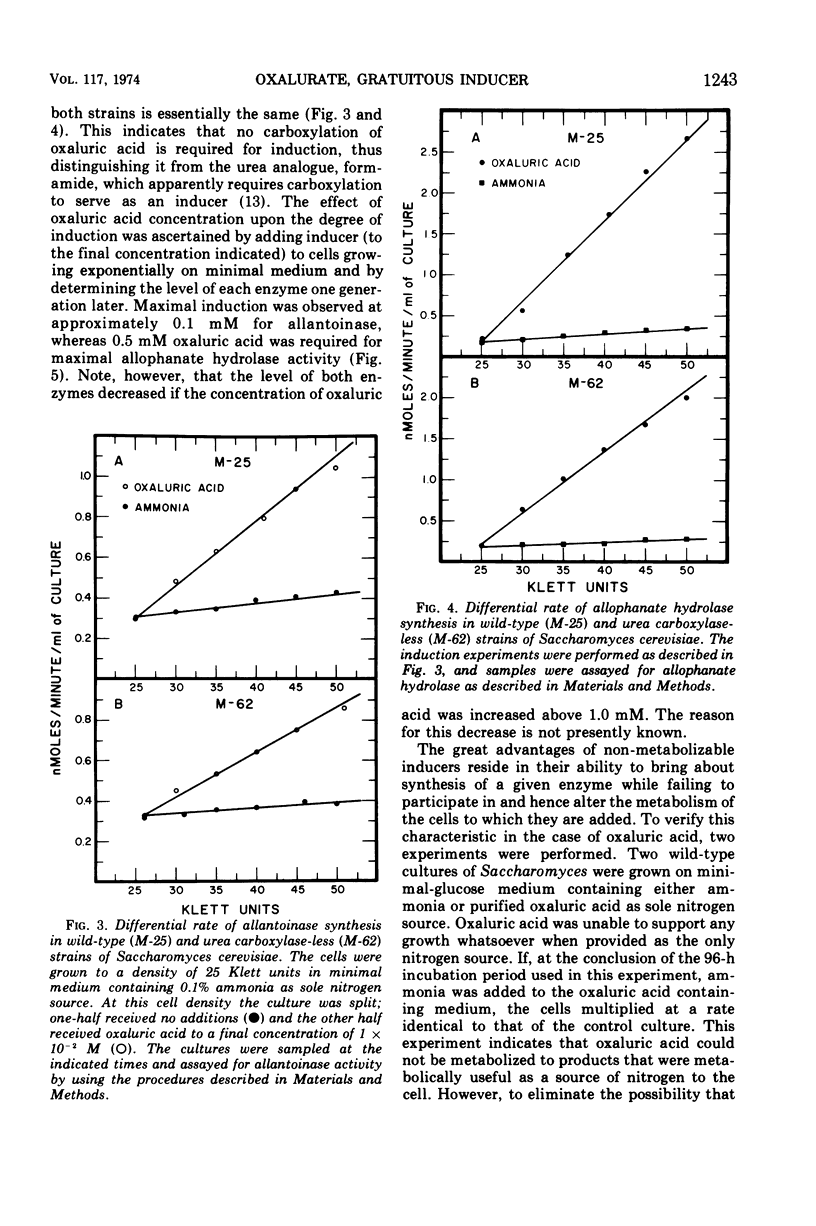
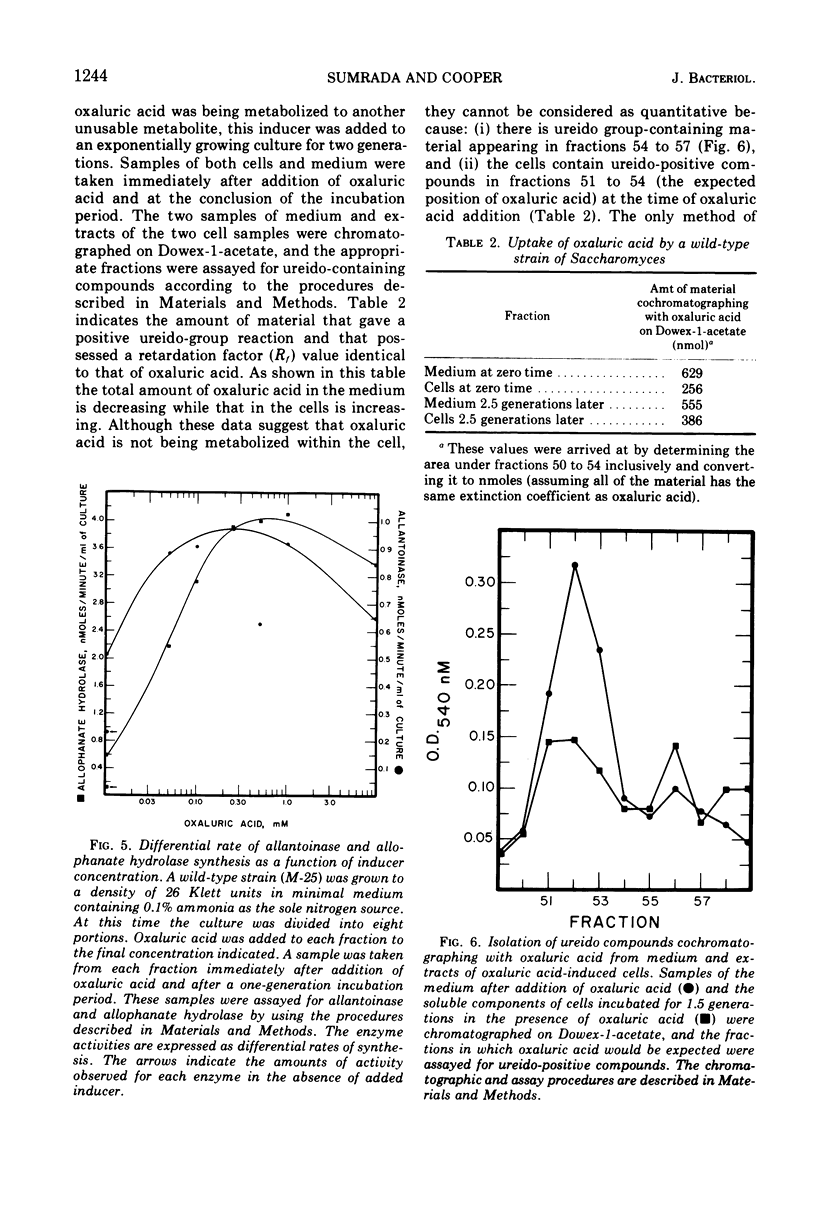
Saccharomyces cerevisiae degrades allantoin in five steps to ammonia, CO2, and glyoxylate. Previously we demonstrated that allophanic acid, the last intermediate of the pathway, was required for induction of all five degradative enzymes. The data presented here indicate that oxaluric acid, an allophanate analogue, is capable of serving as a non-metabolizable inducer. Oxaluric acid brings about a high level of induction even in strains lacking urea carboxylase. Induction observed with parabanic acid was found to result from its spontaneous breakdown to oxaluric acid.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREWS J. C., SELL I. T. The properties and interrelationship of oxaluric and parabanic acids. Arch Biochem Biophys. 1955 Jun;56(2):405–411. doi: 10.1016/0003-9861(55)90261-7. [DOI] [PubMed] [Google Scholar]
- BOJANOWSKI R., GAUDY E., VALENTINE R. C., WOLFE R. S. OXAMIC TRANSCARBAMYLASE OF STREPTOCOCCUS ALLANTOICUS. J Bacteriol. 1964 Jan;87:75–80. doi: 10.1128/jb.87.1.75-80.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Lawther R. P. Induction of the allantoin degradative enzymes in Saccharomyces cerevisiae by the last intermediate of the pathway. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2340–2344. doi: 10.1073/pnas.70.8.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Lawther R. Induction of the allantoin degradative enzymes by allophanic acid, the last intermediate of the pathway. Biochem Biophys Res Commun. 1973 May 1;52(1):137–142. doi: 10.1016/0006-291x(73)90965-0. [DOI] [PubMed] [Google Scholar]
- Eron L., Arditti R., Zubay G., Connaway S., Beckwith J. R. An adenosine 3':5'-cyclic monophosphate-binding protein that acts on the transcription process. Proc Natl Acad Sci U S A. 1971 Jan;68(1):215–218. doi: 10.1073/pnas.68.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. D., Reiness G., Zubay G. Purification and DNA-binding properties of the catabolite gene activator protein. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1222–1225. doi: 10.1073/pnas.68.6.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENTINE R. C., BOJANOWSKI R., GAUDY E., WOLFE R. S. Mechanism of the allantoin fermentation. J Biol Chem. 1962 Jul;237:2271–2277. [PubMed] [Google Scholar]
- VALENTINE R. C., WOLFE R. S. Phosphorolysis of carbamyl oxamic acid. Biochim Biophys Acta. 1960 Dec 4;45:389–391. doi: 10.1016/0006-3002(60)91467-0. [DOI] [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G., Magasanik B. The induction of urea carboxylase and allophanate hydrolase in Saccharomyces cerevisiae. J Biol Chem. 1973 Sep 10;248(17):6203–6209. [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G. Urea carboxylase and allophanate hydrolase. Two components of adenosine triphosphate:urea amido-lyase in Saccharomyces cerevisiae. J Biol Chem. 1972 Mar 10;247(5):1349–1353. [PubMed] [Google Scholar]


