Abstract
With whole cells of a hydrogen cyanide-producing bacterium strain C, of the genus Pseudomonas, it was found that the oxygen necessary for the oxidation of glycine to cyanide could be replaced by various artificial electron acceptors. The order of reactivity was: oxygen > phenazine methosulphate > methylene blue > 2,6-dichlorophenolindophenol > ferricyanide. Cyanide production was inhibited by pyrrolnitrin, a well-known inhibitor of many flavine enzymes. The molar ratio of added glycine to cyanide produced was found to be 1.09. With whole bacteria the apparent Km (glycine) for the cyanide production was found to be 5.0 × 10−4 M.
Full text
PDF
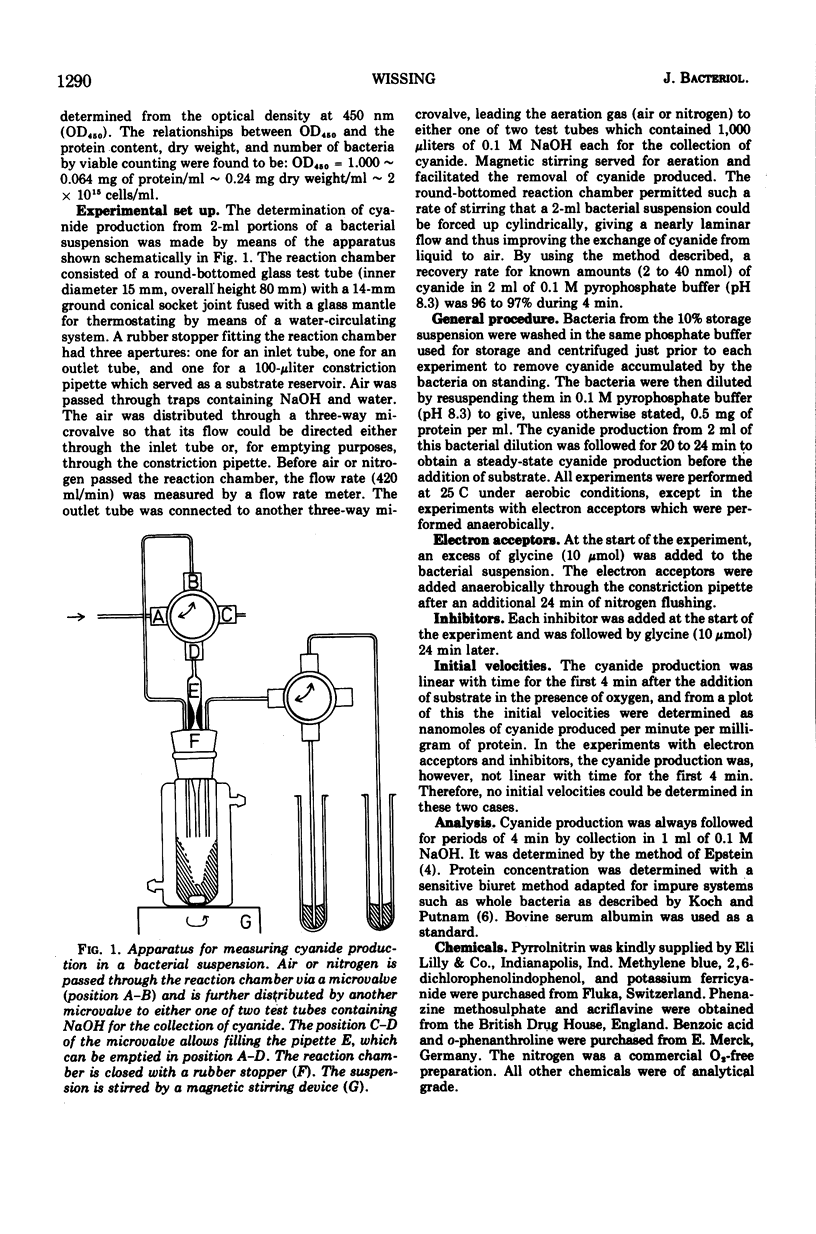
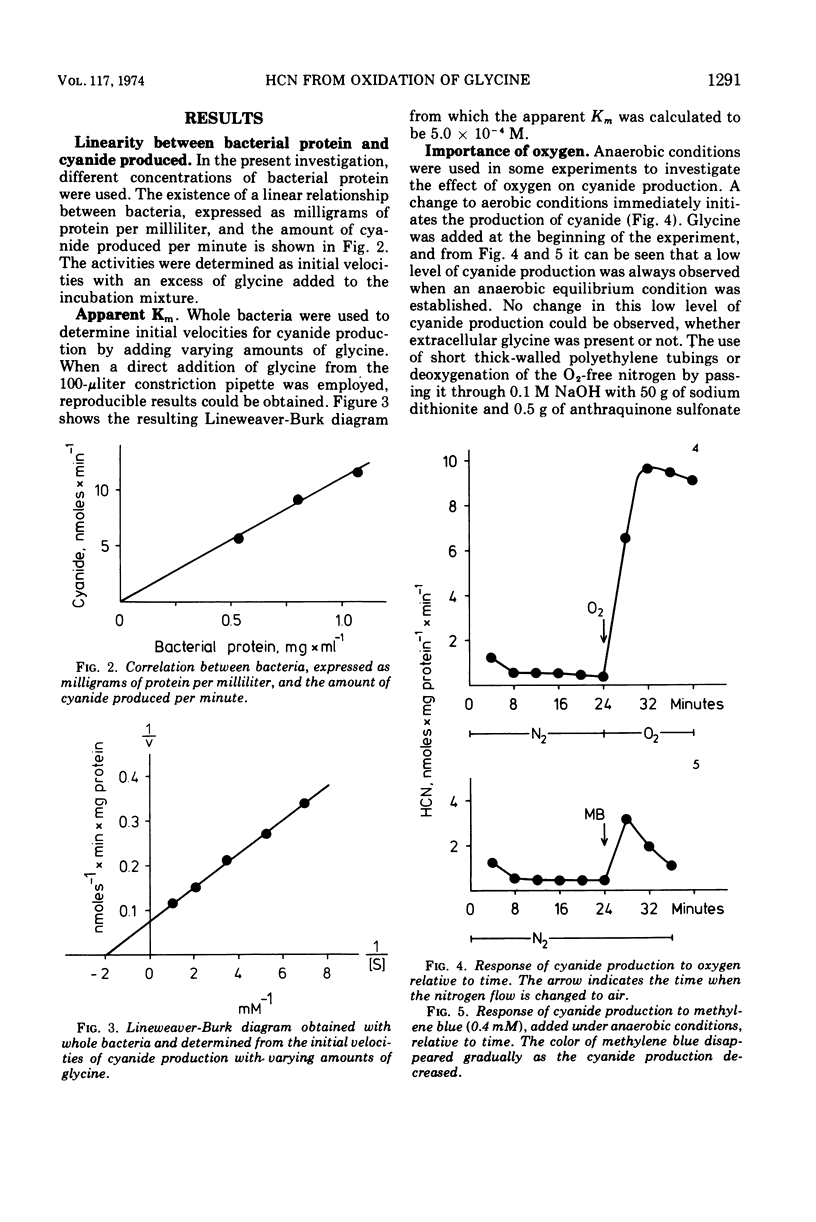
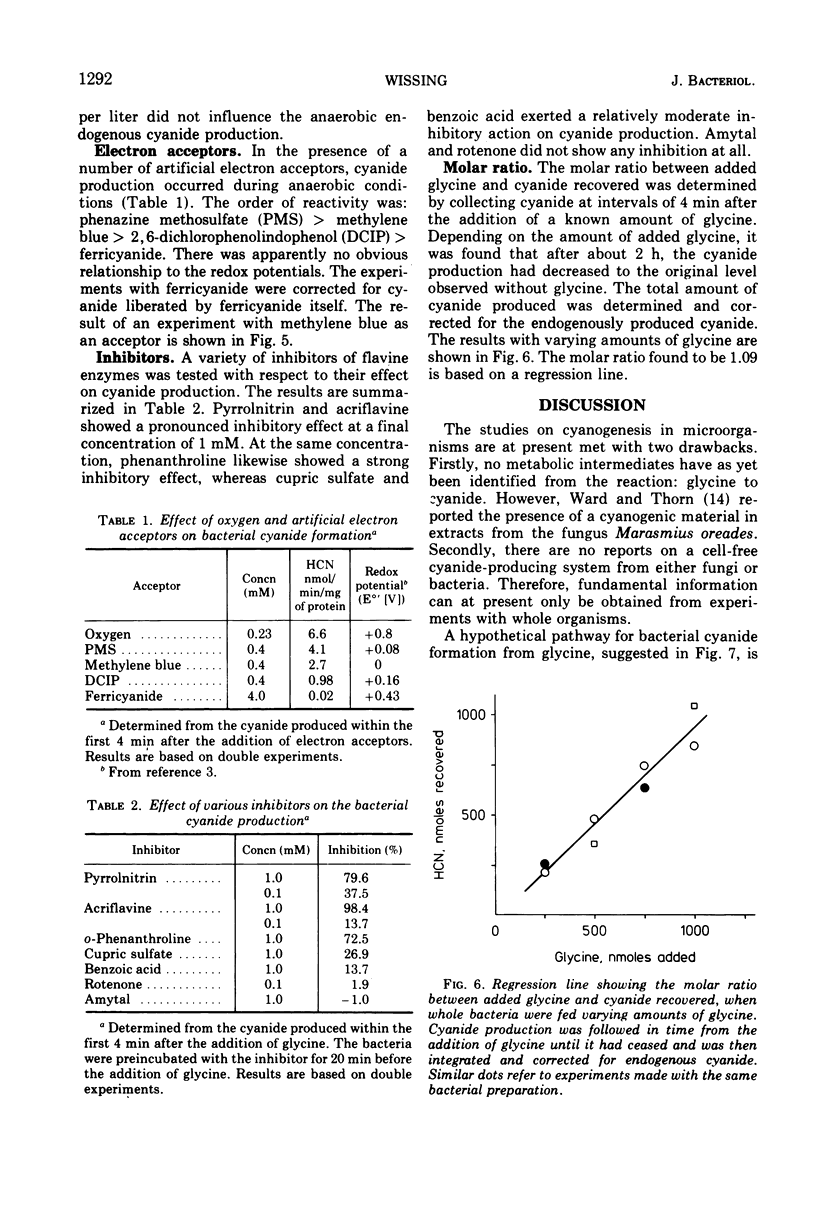


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brysk M. M., Lauinger C., Ressler C. Biosynthesis of cyanide from [2-14C-15N]glycine in Chromobacterium violaceum. Biochim Biophys Acta. 1969 Sep 2;184(3):583–588. doi: 10.1016/0304-4165(69)90272-4. [DOI] [PubMed] [Google Scholar]
- Dixon M. The acceptor specificity of flavins and flavoproteins. 3. Flavoproteins. Biochim Biophys Acta. 1971 Mar 2;226(2):269–284. doi: 10.1016/0005-2728(71)90094-6. [DOI] [PubMed] [Google Scholar]
- Hafner E. W., Wellner D. Demonstration of imino acids as products of the reactions catalyzed by D- and L-amino acid oxidases. Proc Natl Acad Sci U S A. 1971 May;68(5):987–991. doi: 10.1073/pnas.68.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUBO H., YAMANO T., IWATSUBO M., WATARI H., SHIGA T., ISOMOTO A. [On the crystallization and the purification of D-amino acid oxidase]. Bull Soc Chim Biol (Paris) 1960;42:569–582. [PubMed] [Google Scholar]
- Koch A. L., Putnam S. L. Sensitive biuret method for determination of protein in an impure system such as whole bacteria. Anal Biochem. 1971 Nov;44(1):239–245. doi: 10.1016/0003-2697(71)90366-6. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M., Slayman C. W. Effect of pyrrolnitrin on electron transport and oxidative phosphorylation in mitochondria isolated from Neurospora crassa. J Bacteriol. 1972 Nov;112(2):1020–1022. doi: 10.1128/jb.112.2.1020-1022.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels R., Hankes L. V., Corpe W. A. Cyanide formation from glycine by nonproliferating cells of Chromobacterium violaceum. Arch Biochem Biophys. 1965 Jul;111(1):121–125. doi: 10.1016/0003-9861(65)90329-2. [DOI] [PubMed] [Google Scholar]
- NEIMS A. H., HELLERMAN L. Specificity of the D-amino acid oxidase in relation to glycine oxidase activity. J Biol Chem. 1962 Mar;237:PC976–PC978. [PubMed] [Google Scholar]
- Tripathi R. K., Gottlieb D. Mechanism of action of the antifungal antibiotic pyrrolnitrin. J Bacteriol. 1969 Oct;100(1):310–318. doi: 10.1128/jb.100.1.310-318.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


