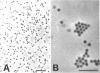
Abstract
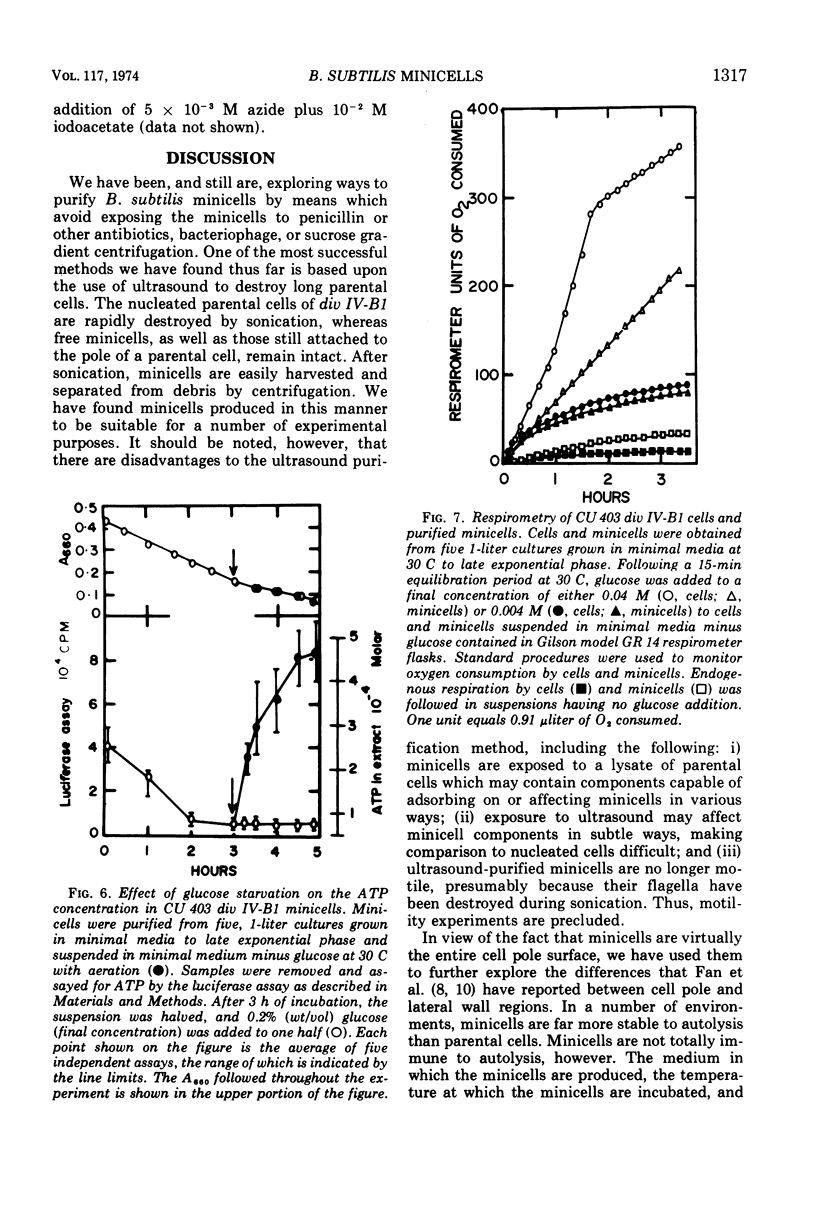
Minicells produced by Bacillus subtilis strains carrying the div IV-B1 mutation, (CU 403 div IV-B1 and CU 403 div IV-B1, tag-1), were purified by a procedure which destroys parental cells with ultrasound, but spares minicells. Such preparations generally contain 109 or more minicells/ml and less than 104 colony-forming units/ml. Purified minicells are resistant to autolysis in tris(hydroxymethyl)aminomethane buffer, pH 7.5, at 30 C, conditions which result in total lysis of parental cells. Minicells are not completely devoid of autolytic activity, however. The medium in which minicells are produced, the temperature at which purified minicells are incubated, and the genotype of cells from which the minicells are derived all influence the rate of autolysis of purified minicells. These parameters are demonstrated by using minicells obtained from div IV-B1 and div IV-B1, tag-1 strains. Ultrastructural differences have been observed in the products of autolysis of these two minicell strains. Minicells are sensitive to low levels of lysozyme and yield miniprotoplasts when the wall is removed in an osmotically protective environment. Although minicells are unable to grow, they can maintain their integrity over long periods of time, which suggests functional energy metabolism in minicells. Direct measurements of adenosine 5′-triphosphate (ATP) levels by the luciferase assay indicated that minicells can produce ATP. Oxygen consumption, measured by standard respirometry techniques, also indicates functional metabolism in minicells. These findings demonstrate that minicells purified by ultrasound are suitable material for study of physiological processes in anucleate cells.
Full text
PDF

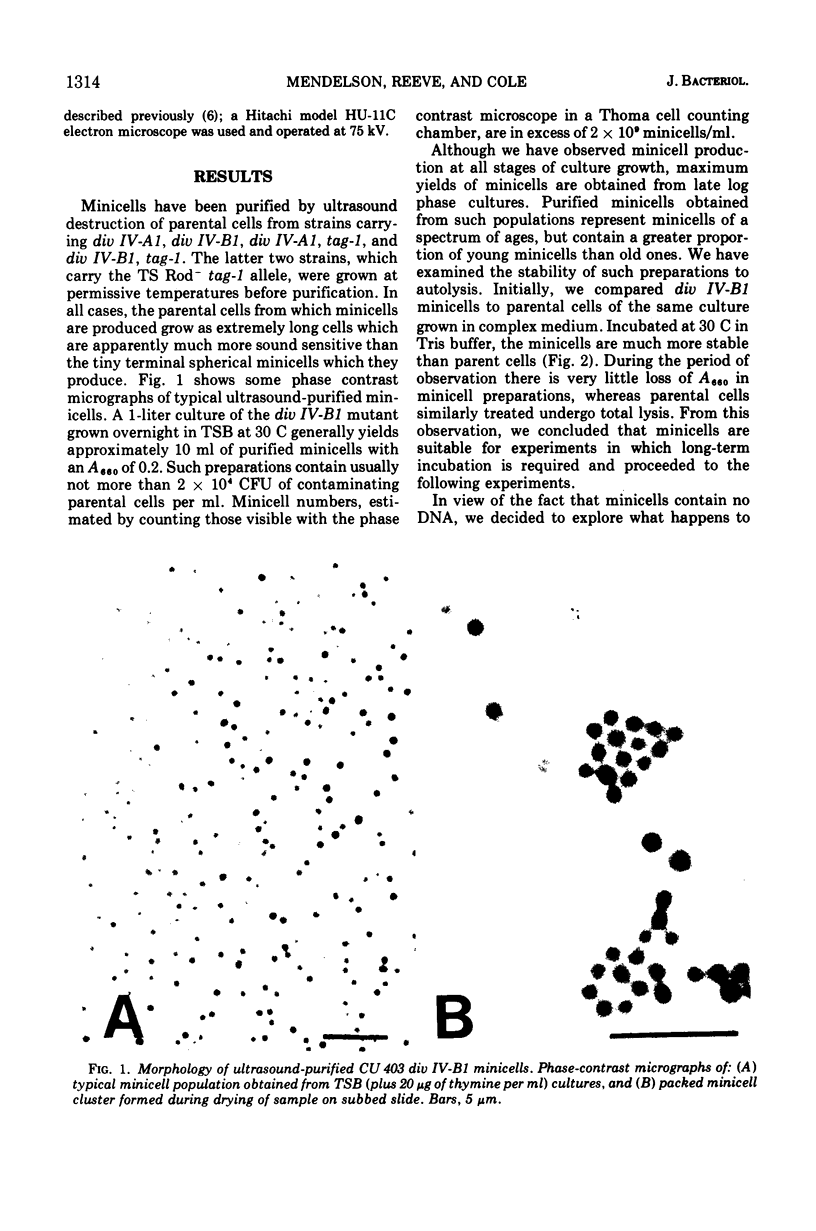
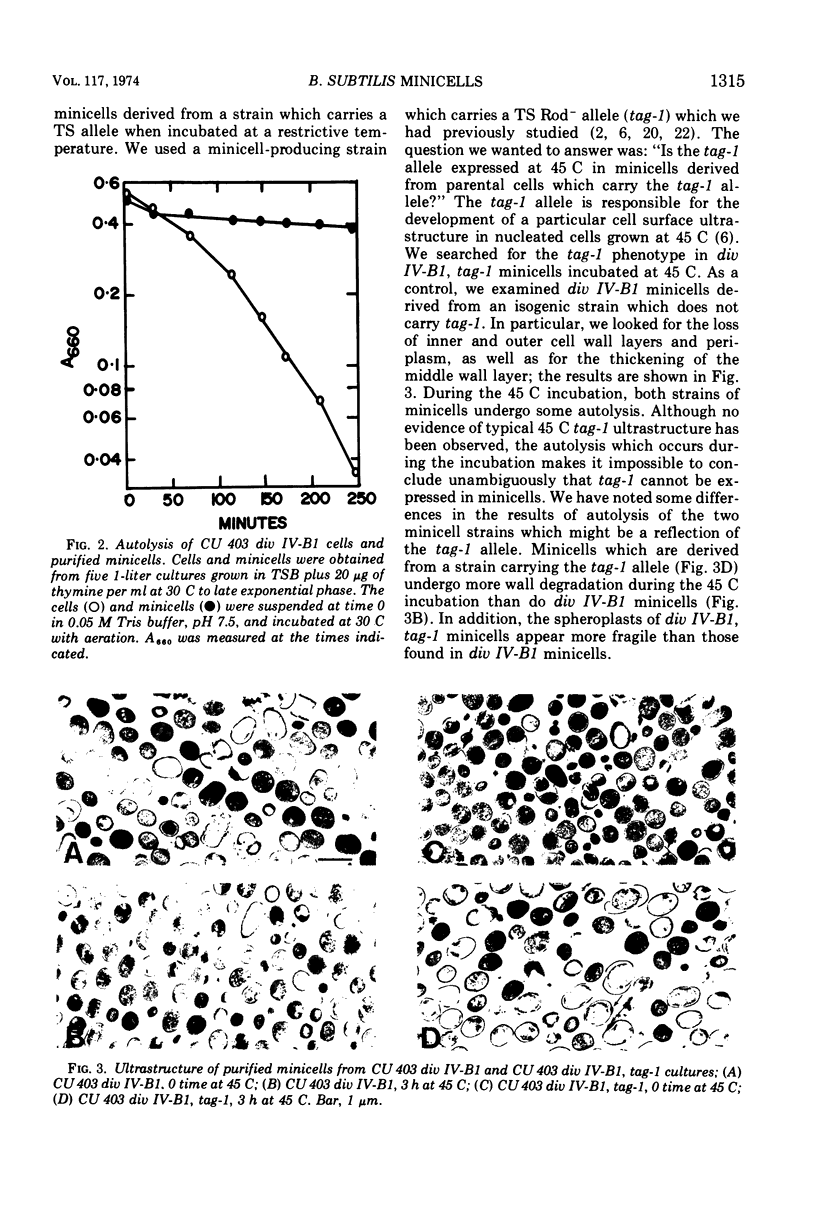
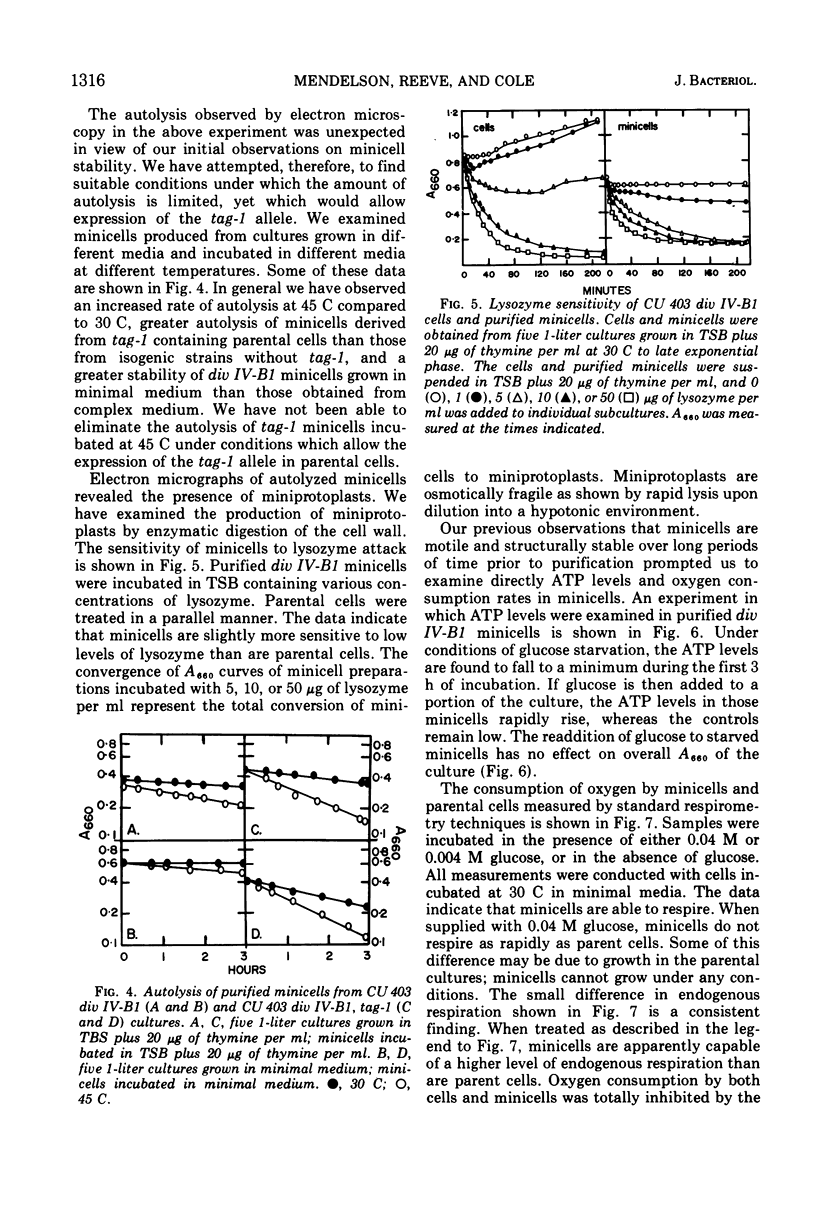


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan R. J., Mendelson N. H., Brooks D., Young F. E. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972 Apr;110(1):281–290. doi: 10.1128/jb.110.1.281-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLIFTON C. E. Endogenous metabolism and oxidative assimilation of typical bacterial species. Ann N Y Acad Sci. 1963 Jan 21;102:655–668. doi: 10.1111/j.1749-6632.1963.tb13666.x. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipman D. M., Sharon N. Mechanism of lysozyme action. Science. 1969 Aug 1;165(3892):454–465. doi: 10.1126/science.165.3892.454. [DOI] [PubMed] [Google Scholar]
- Cole R. M., Popkin T. J., Boylan R. J., Mendelson N. H. Ultrastructure of a temperature-sensitive rod- mutant of Bacillus subtilis. J Bacteriol. 1970 Sep;103(3):793–810. doi: 10.1128/jb.103.3.793-810.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrati-Elizur E. Spontaneous transformation in Bacillus subtilis. Genet Res. 1968 Feb;11(1):83–96. doi: 10.1017/s0016672300011216. [DOI] [PubMed] [Google Scholar]
- Fan D. P., Beckman B. E. Structural difference between walls from hemispherical caps and partial septa of Bacillus subtilis. J Bacteriol. 1973 May;114(2):790–797. doi: 10.1128/jb.114.2.790-797.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M., Cunningham W. P. Ultrastructural studies on a mutant of Bacillus subtilis whose growth is inhibited due to insufficient autolysin production. J Bacteriol. 1972 Mar;109(3):1247–1257. doi: 10.1128/jb.109.3.1247-1257.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Pelvit M. C., Cunningham W. P. Structural difference between walls from ends and sides of the rod-shaped bacterium Bacillus subtilis. J Bacteriol. 1972 Mar;109(3):1266–1272. doi: 10.1128/jb.109.3.1266-1272.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C., Rogers H. J. Autolytic enzymes in growth of bacteria. Nature. 1971 Jan 22;229(5282):272–273. doi: 10.1038/229272a0. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Khachatourians G. G., Saunders C. A. A new method for the preparation of minicells for physiological studies. Prep Biochem. 1973;3(3):291–298. doi: 10.1080/00327487308061513. [DOI] [PubMed] [Google Scholar]
- MALLETTE M. F. Validity of the concept of energy of maintenance. Ann N Y Acad Sci. 1963 Jan 21;102:521–535. doi: 10.1111/j.1749-6632.1963.tb13658.x. [DOI] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- McGrew S. B., Mallette M. F. ENERGY OF MAINTENANCE IN ESCHERICHIA COLI. J Bacteriol. 1962 Apr;83(4):844–850. doi: 10.1128/jb.83.4.844-850.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H., Reeve J. N. Growth of the Bacillus subtilis cell surface. Nat New Biol. 1973 May 9;243(123):62–64. [PubMed] [Google Scholar]
- Reeve J. N., Mendelson N. H., Coyne S. I., Hallock L. L., Cole R. M. Minicells of Bacillus subtilis. J Bacteriol. 1973 May;114(2):860–873. doi: 10.1128/jb.114.2.860-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Pooley H. M., Thompson J. S. Autolytic enzyme system of Streptococcus faecalis. 3. Localization of the autolysin at the sites of cell wall synthesis. J Bacteriol. 1967 Nov;94(5):1525–1530. doi: 10.1128/jb.94.5.1525-1530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Autolytic enzyme associated with cell walls of Bacillus subtilis. J Biol Chem. 1966 Aug 10;241(15):3462–3467. [PubMed] [Google Scholar]