Abstract
The dextransucrase (EC 2.4.1.5) activity from cell-free culture supernatants of Streptococcus mutans strain 6715 has been purified approximately 1,500-fold by ammonium sulfate precipitation, hydroxylapatite chromatography, and isoelectric focusing. The enzyme was eluted as a single peak of activity from hydroxylapatite, and isoelectric focusing of the resulting preparation gave a single band of dextransucrase activity which focused at a pH of 4.0. The final enzyme preparation contained two distinct, enzymatically active proteins as judged by assay in situ after polyacrylamide gel electrophoresis. One of the proteins represented 90% of the total dextransucrase activity and 53% of the total protein. The molecular weight of the enzyme was estimated by gel filtration to be 94,000. The temperature optimum of the enzyme was broad (34 to 42 C) and its pH range was rather narrow, with optimal activity at pH 5.5. The Km for sucrose was 3 mM, and fructose competitively inhibited the enzyme reaction with a Ki of 27 mM.
Full text
PDF

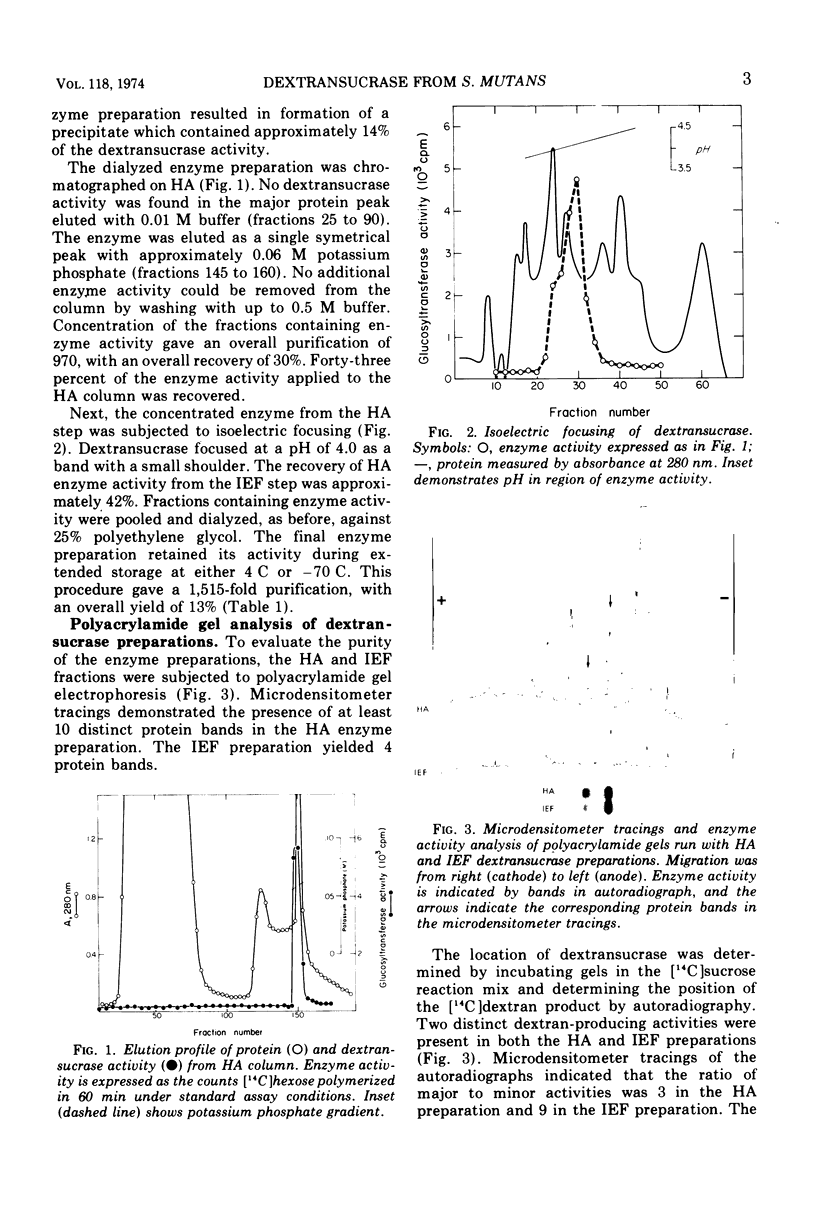
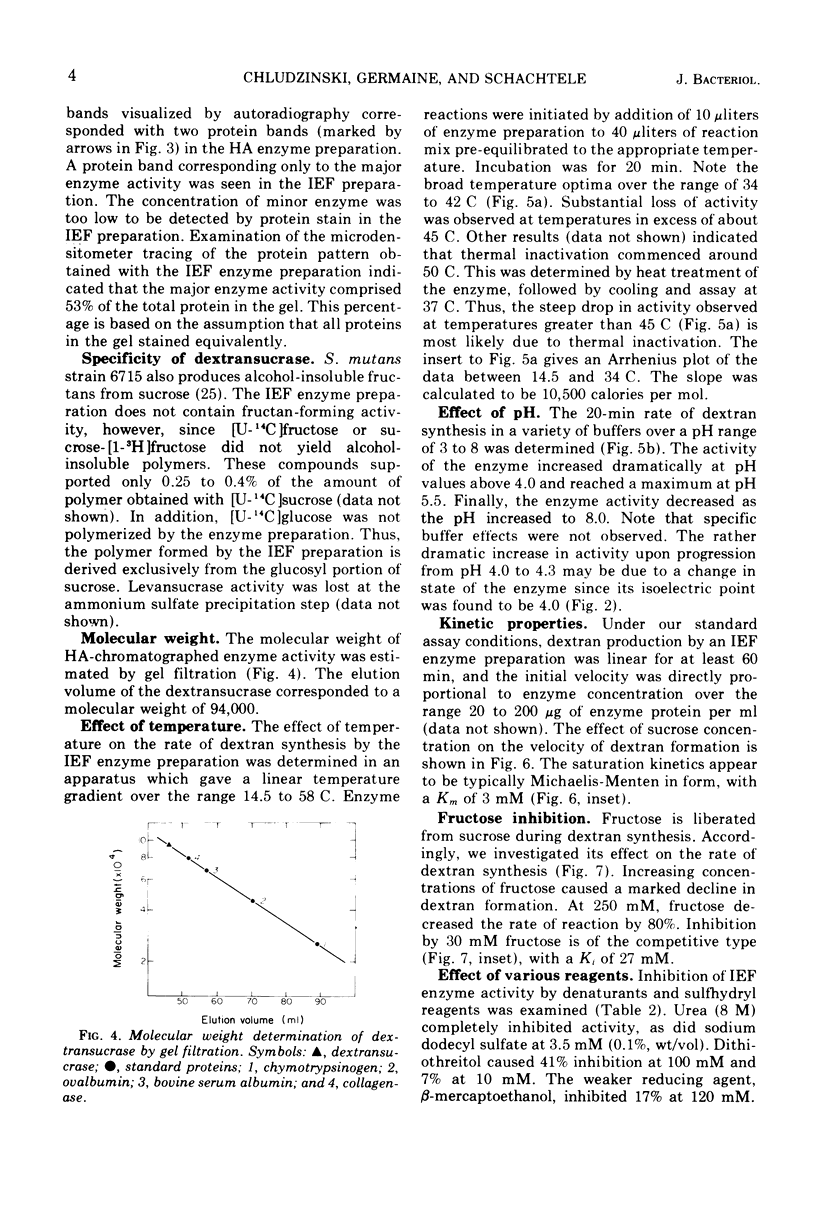
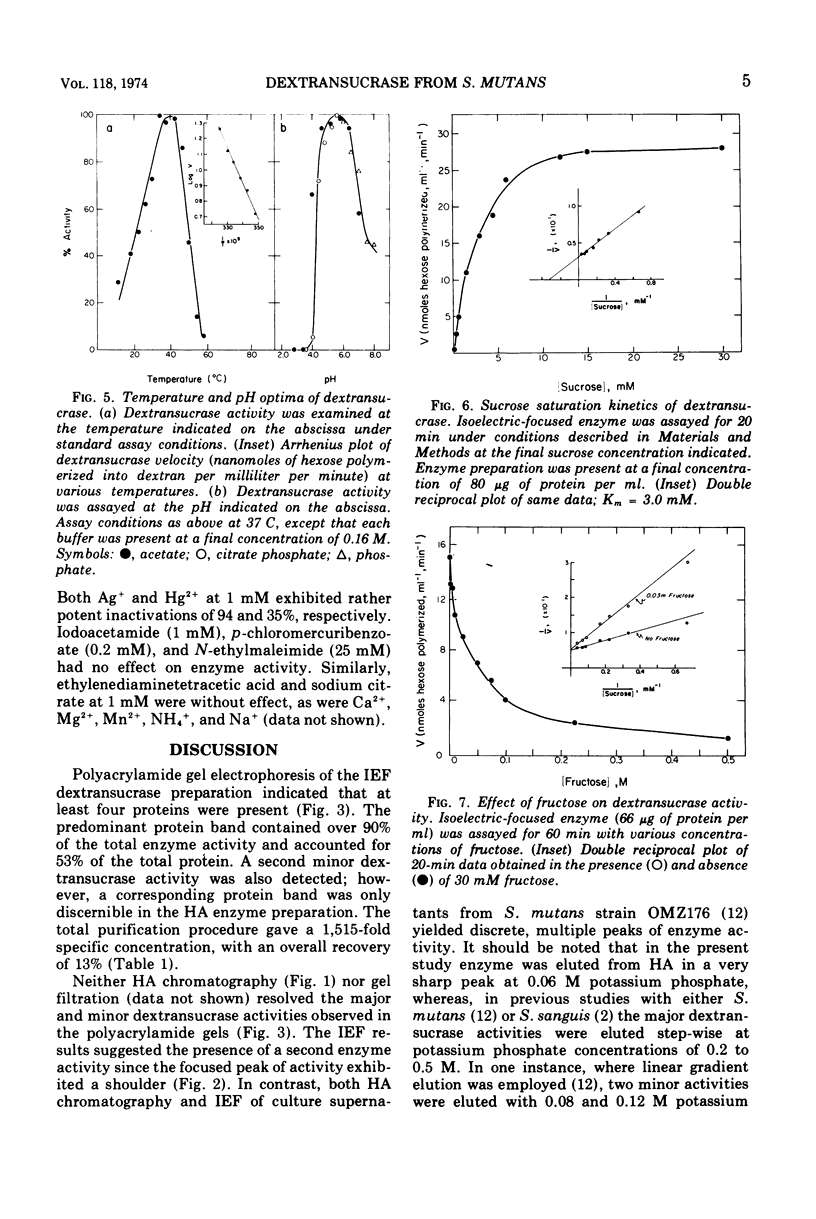


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY R. W. Transglucosidase activity of rumen strains of Streptococcus bovis. 2. Isolation and properties of dextransucrase. Biochem J. 1959 May;72(1):42–49. doi: 10.1042/bj0720042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Newbrun E., Krasse B. Purification and properties of dextransucrase from Streptococcus sanguis. Arch Oral Biol. 1969 May;14(5):469–478. doi: 10.1016/0003-9969(69)90140-x. [DOI] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer Dirks O. The relationship between extracellular polysaccharide-producing streptococci and smooth surface caries in 13-year-old children. Caries Res. 1969;3(2):190–199. doi: 10.1159/000259582. [DOI] [PubMed] [Google Scholar]
- Ebert K. H., Brosche M. Origin of branches in native dextrans. Biopolymers. 1967 Jun;5(5):423–429. doi: 10.1002/bip.1967.360050503. [DOI] [PubMed] [Google Scholar]
- Ebert K. H., Schenk G. Mechanisms of biopolymer growth: the formation of dextran and levan. Adv Enzymol Relat Areas Mol Biol. 1968;30:179–221. doi: 10.1002/9780470122754.ch4. [DOI] [PubMed] [Google Scholar]
- Fitzgerlad R. J. Dental caries research in gnotobiotic animals. Caries Res. 1968;2(2):139–146. doi: 10.1159/000259552. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. B. Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol. 1967 Jan;12(1):11–23. doi: 10.1016/0003-9969(67)90137-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Guggenheim B. Enzymatic hydrolysis and structure of water-insoluble glucan produced by glucosyltransferases from a strain of streptococcus mutans. Helv Odontol Acta. 1970 Nov;14(Suppl):89+–89+. [PubMed] [Google Scholar]
- Guggenheim B., Newbrun E. Extracellular glucosyltransferase activity of an HS strain of Streptococcus mutans. Helv Odontol Acta. 1969 Oct;13(2):84–97. [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Biochemical and morphological aspects of extracellular polysaccharides produced by cariogenic streptococci. Helv Odontol Acta. 1967 Oct;11(2):131–152. [PubMed] [Google Scholar]
- Keyes P. H. Research in dental caries. J Am Dent Assoc. 1968 Jun;76(6):1357–1373. doi: 10.14219/jada.archive.1968.0186. [DOI] [PubMed] [Google Scholar]
- Krasse B., Jordan H. V., Edwardsson S., Svensson I., Trell L. The occurrence of certain "caries-inducing" streptococci in human dental plaque material with special reference to frequency and activity of caries. Arch Oral Biol. 1968 Aug;13(8):911–918. doi: 10.1016/0003-9969(68)90006-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Long L. W., Edwards J. R. Detailed structure of a dextran from a cariogenic bacterium. Carbohydr Res. 1972 Sep;24(1):216–217. doi: 10.1016/s0008-6215(00)82285-5. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Origin of the cell-associated dextransucrase of Streptococcus mutans. Infect Immun. 1973 Jun;7(6):829–838. doi: 10.1128/iai.7.6.829-838.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbrun E., Carlsson J. Reaction rate of dextransucrase from Streptococcus sanguis in the presence of various compounds. Arch Oral Biol. 1969 May;14(5):461–468. doi: 10.1016/0003-9969(69)90139-3. [DOI] [PubMed] [Google Scholar]
- Robrish S. A., Reid W., Krichevsky M. I. Distribution of enzymes forming polysaccharide from sucrose and the composition of extracellular polysaccharide synthesized by Streptococcus mutans. Appl Microbiol. 1972 Aug;24(2):184–190. doi: 10.1128/am.24.2.184-190.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Loken A. E., Knudson D. J. Preferential utilization of the glucosyl moiety of sucrose by a cariogenic strain of Streptococcus mutans. Infect Immun. 1972 Apr;5(4):531–536. doi: 10.1128/iai.5.4.531-536.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Loken A. E., Schmitt M. K. Use of specifically labeled sucrose for comparison of extracellular glucan and fructan metabolism by oral streptococci. Infect Immun. 1972 Feb;5(2):263–266. doi: 10.1128/iai.5.2.263-266.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Critchley P. The extracellular polysaccharide produced from sucrose by a cariogenic streptococcus. Arch Oral Biol. 1966 Oct;11(10):1039–1042. doi: 10.1016/0003-9969(66)90204-4. [DOI] [PubMed] [Google Scholar]