Abstract
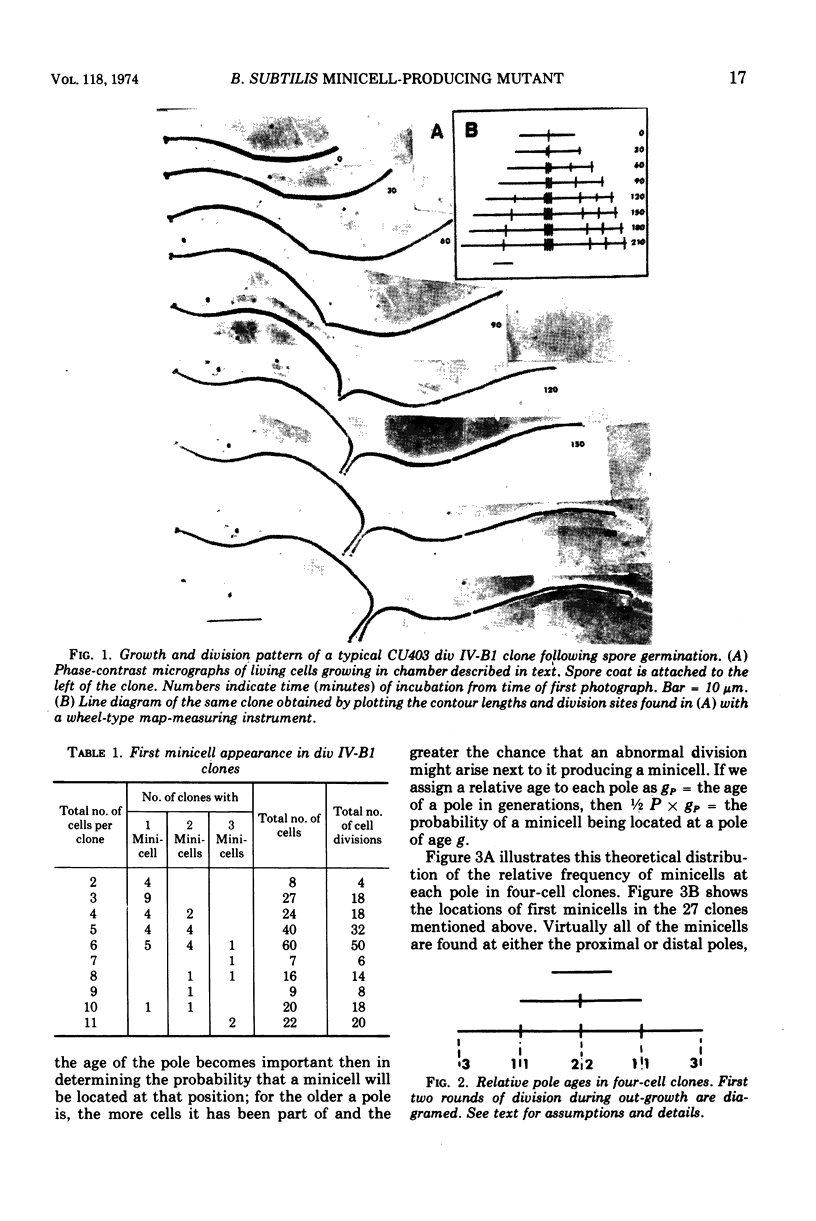
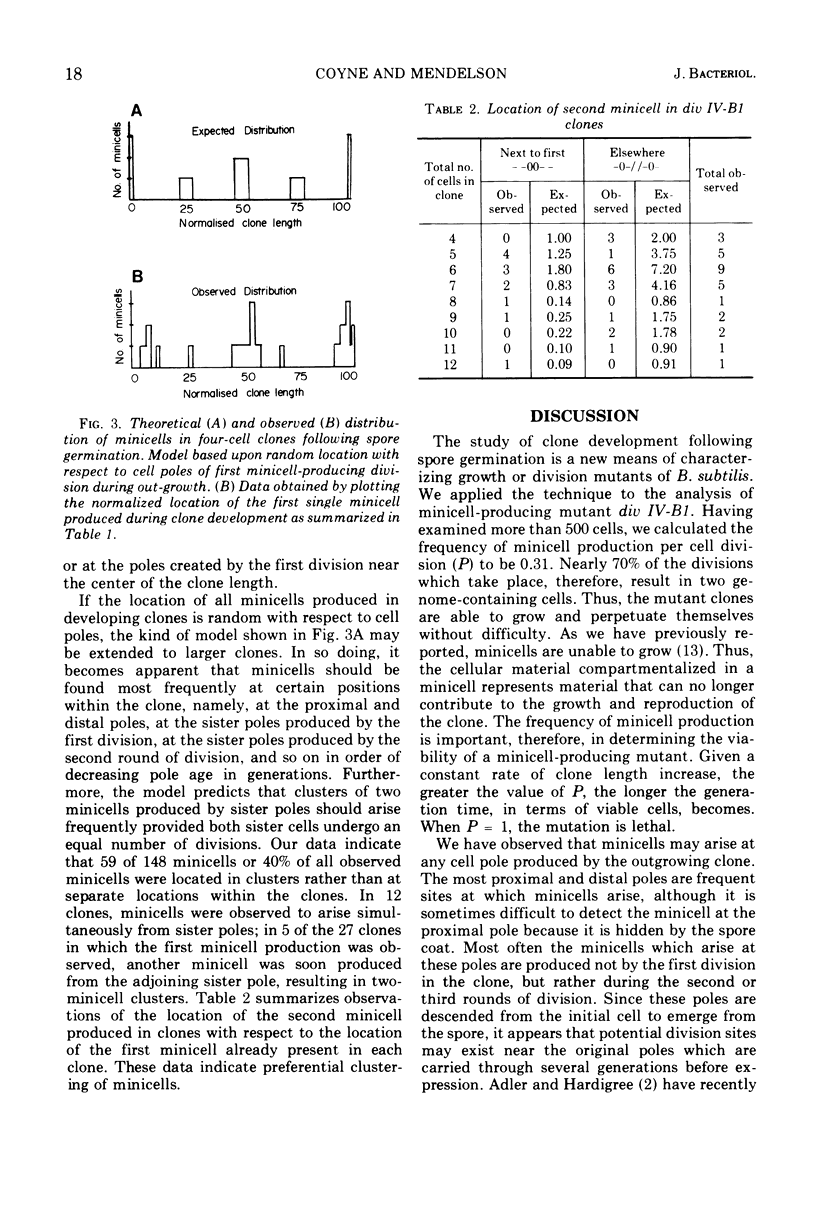
Spores of the Bacillus subtilis minicell-producing mutant div IV-B1 were germinated and grown to microcolonies in chambers which facilitate continuous observation of the developing clones with a phase-contrast microscope. Time lapse photographs were taken of 46 clones, covering the period from the beginning of outgrowth until at least two rounds of cell division had been completed. Cell lineages were constructed from contour length measurements of the photographs. These data include cell lengths, division site locations, and cell numbers in clones of various ages. From these data we have determined that the probability of a minicell being produced at any division by the div IV-B1 mutant is 0.31. The location of the abnormal division site which generates the first minicell produced in the outgrowing clone appears to be random with respect to the existing cell poles. In contrast, the location of the second abnormal division site, and hence the second minicell, is not random but rather occurs preferentially in proximity to the first minicell. This clustering of abnormal events suggests that division site location is related to pole age (generations), although other influences on minicell clustering cannot be ruled out at present.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balassa G. The genetic control of spore formation in bacilli. Curr Top Microbiol Immunol. 1971;56:99–192. doi: 10.1007/978-3-642-65241-7_4. [DOI] [PubMed] [Google Scholar]
- Boylan R. J., Mendelson N. H. Initial characterization of a temperature-sensitive rod--mutant of Bacillus subtilis. J Bacteriol. 1969 Dec;100(3):1316–1321. doi: 10.1128/jb.100.3.1316-1321.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Fisher W. D., Curtiss R., 3rd, Adler H. I. The properties of DNA transferred to minicells during conjugation. Cold Spring Harb Symp Quant Biol. 1968;33:635–641. doi: 10.1101/sqb.1968.033.01.071. [DOI] [PubMed] [Google Scholar]
- Inselburg J. Segregation into and replication of plasmid deoxyribonucleic acid in chromosomeless segregants of Escherichia coli. J Bacteriol. 1970 Jun;102(3):642–647. doi: 10.1128/jb.102.3.642-647.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson G. M. Ability of CBA mice to produce anti-idiotypic sera to 5563 myeloma protein. Nature. 1970 Jul 18;227(5255):273–274. doi: 10.1038/227273a0. [DOI] [PubMed] [Google Scholar]
- Mendelson N. H., Cole R. M. Genetic regulation of cell division initiation in Bacillus subtilis. J Bacteriol. 1972 Nov;112(2):994–1003. doi: 10.1128/jb.112.2.994-1003.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H., Reeve J. N. Growth of the Bacillus subtilis cell surface. Nat New Biol. 1973 May 9;243(123):62–64. [PubMed] [Google Scholar]
- Paulton R. J. Analysis of the multiseptate potential of Bacillus subtilis. J Bacteriol. 1970 Nov;104(2):762–767. doi: 10.1128/jb.104.2.762-767.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulton R. J. Nuclear and cell division in filamentous bacteria. Nat New Biol. 1971 Jun 30;231(26):271–274. doi: 10.1038/newbio231271a0. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Mendelson N. H., Coyne S. I., Hallock L. L., Cole R. M. Minicells of Bacillus subtilis. J Bacteriol. 1973 May;114(2):860–873. doi: 10.1128/jb.114.2.860-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Mendelson N. H. Pronase digestion of amino-acid binding components on the surface of Bacillus subtilis cells and minicells. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1325–1330. doi: 10.1016/0006-291x(73)90610-4. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Allison D. P., Beattie K. L. Relationship between prophage induction and transformation in Haemophilus influenzae. J Bacteriol. 1973 Jul;115(1):153–161. doi: 10.1128/jb.115.1.153-161.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy R. J., Allison D. P., Curtiss R., 3rd Cryptic plasmids in a minicell-producing strain of Salmonella typhimurium. J Bacteriol. 1973 Apr;114(1):439–442. doi: 10.1128/jb.114.1.439-442.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy R. J., Perry A., Allison D. P., Curtiss R., 3rd Molecular nature of R-factor deoxyribonucleic acid isolated from Salmonella typhimurium minicells. J Bacteriol. 1973 Jun;114(3):1328–1335. doi: 10.1128/jb.114.3.1328-1335.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]