Abstract
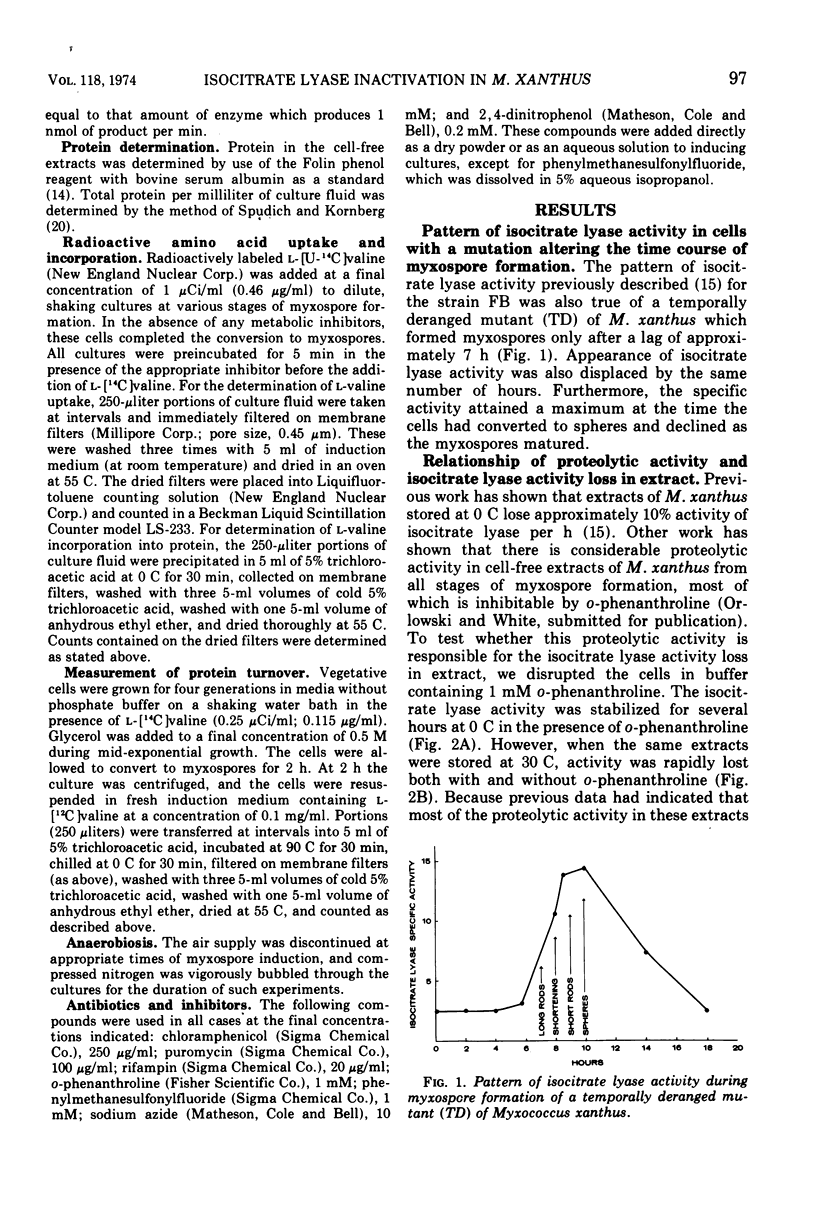
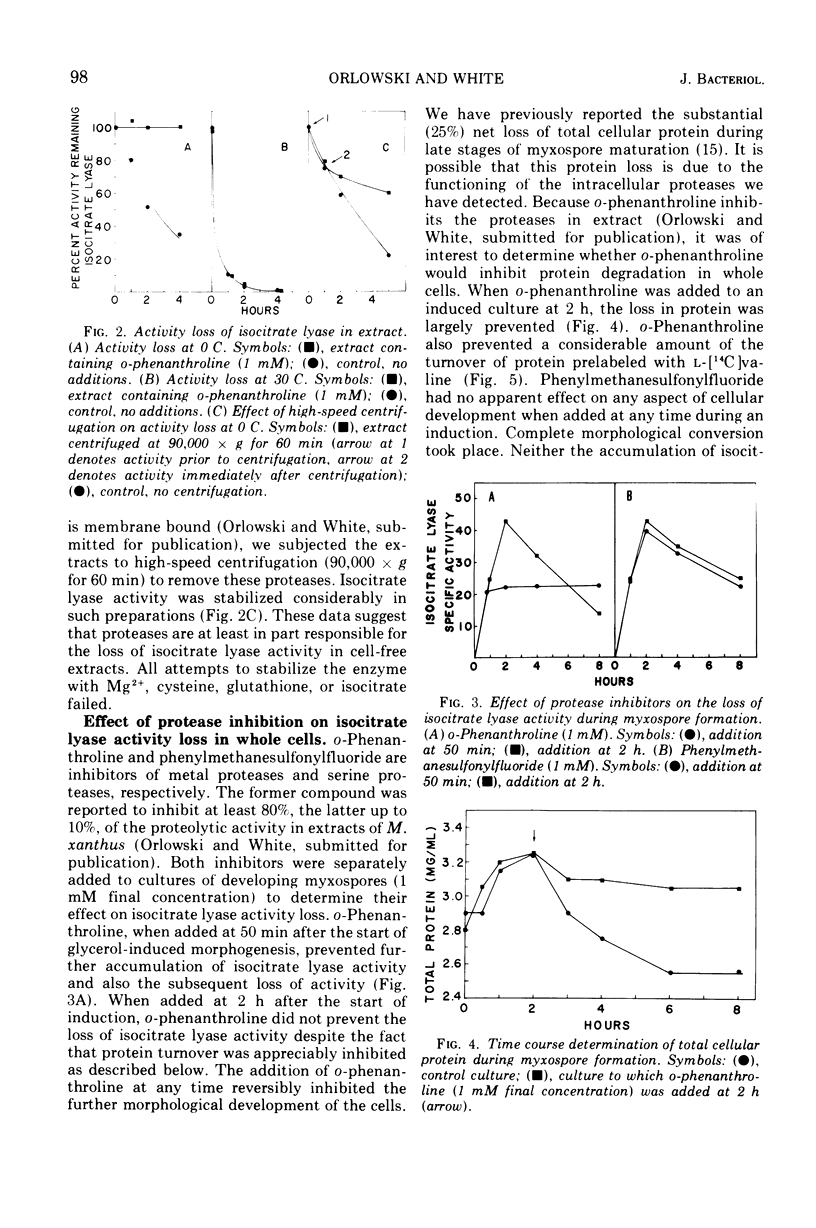
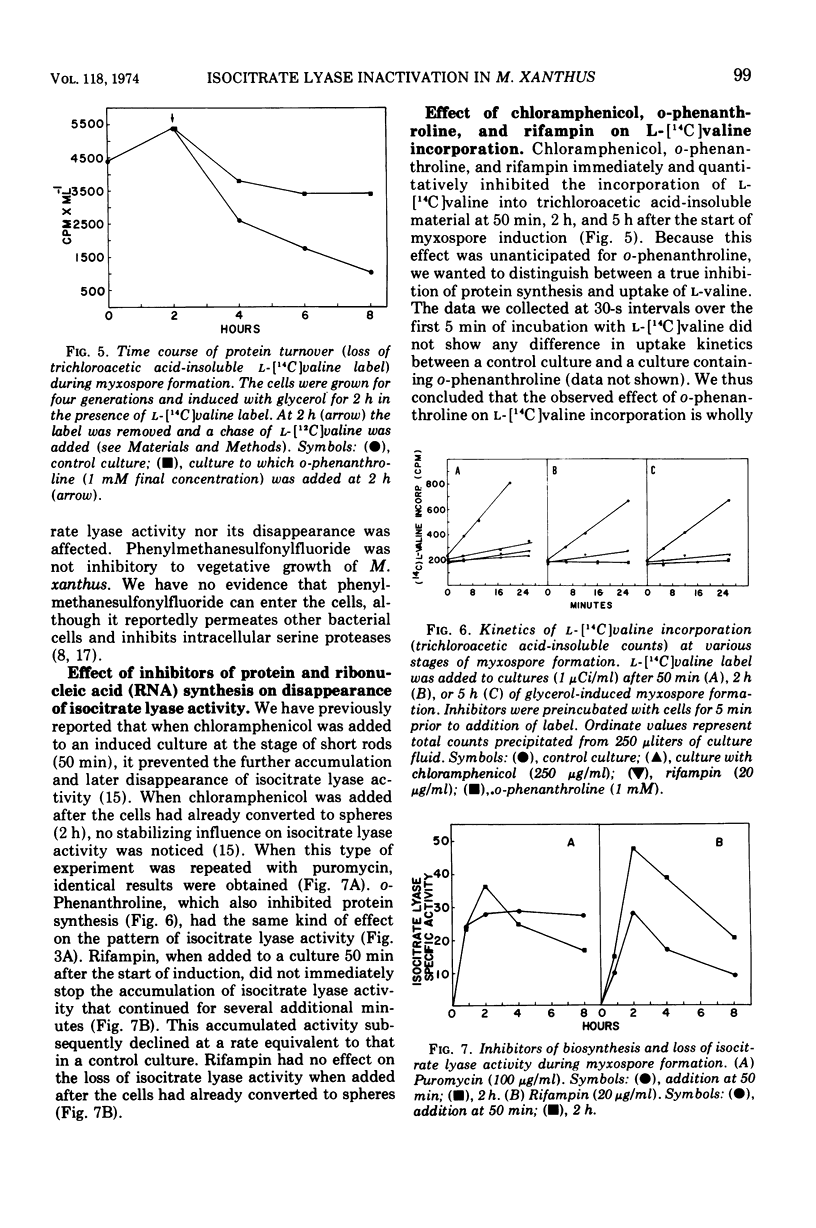
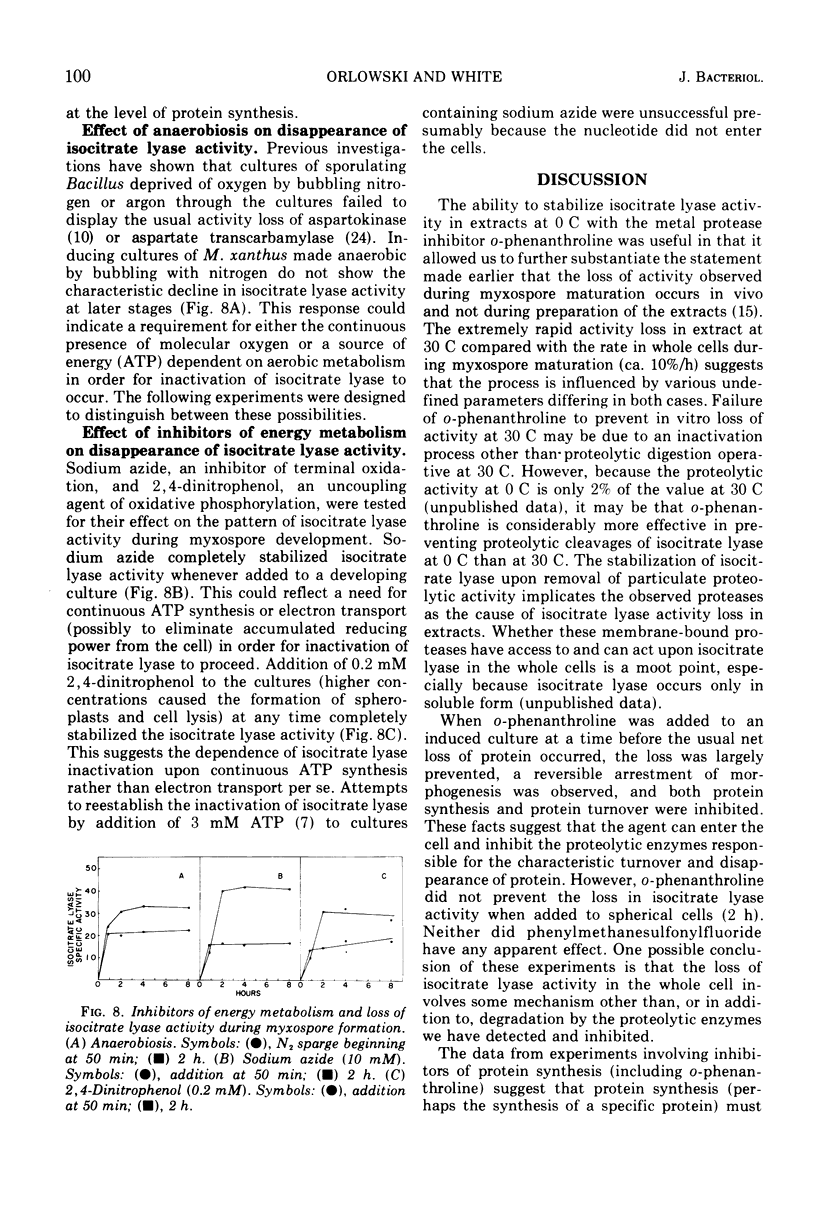
The inactivation of isocitrate lyase which occurs during late stages of myxospore formation in Myxococcus xanthus was studied. Several findings are reported. (i) Protein synthesis is required over a specific time interval in order for isocitrate lyase inactivation to occur at a later time. (ii) Metabolic energy is required at all times during myxospore development if the inactivation is to occur. (iii) It was possible to inhibit protein turnover to a considerable extent without affecting the net loss in isocitrate lyase activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. Regulation of enzyme function. Annu Rev Microbiol. 1969;23:47–68. doi: 10.1146/annurev.mi.23.100169.000403. [DOI] [PubMed] [Google Scholar]
- Bland J., Yeh W. K., White D., Hendricks A. Increase in glyoxylate shunt enzymes during cellular morphogenesis in Myxococcus xanthus. Can J Microbiol. 1971 Feb;17(2):209–211. doi: 10.1139/m71-036. [DOI] [PubMed] [Google Scholar]
- Chulavatnatol M., Atkinson D. E. Phosphoenolpyruvate synthetase from Escherichia coli. Effects of adenylate energy charge and modifier concentrations. J Biol Chem. 1973 Apr 25;248(8):2712–2715. [PubMed] [Google Scholar]
- DWORKIN M., GIBSON S. M. A SYSTEM FOR STUDYING MICROBIAL MORPHOGENESIS: RAPID FORMATION OF MICROCYSTS IN MYXOCOCCUS XANTHUS. Science. 1964 Oct 9;146(3641):243–244. doi: 10.1126/science.146.3641.243. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. 8. Patterns of enzyme development during growth and sporulation of Baccillus subtilis. J Biol Chem. 1968 Sep 25;243(18):4653–4660. [PubMed] [Google Scholar]
- Dworkin M., Sadler W. Induction of cellular morphogenesis in Myxococcus xanthus. I. General description. J Bacteriol. 1966 Apr;91(4):1516–1519. doi: 10.1128/jb.91.4.1516-1519.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdos A., Gajdos-Török M., Gorchein A., Neuberger A., Tait G. H. The effect of adenosine triphosphate on porphyrin excretion and on glycine metabolism in Rhodopseudomonas spheroides. Biochem J. 1968 Jan;106(1):185–192. doi: 10.1042/bj1060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin). Proc Natl Acad Sci U S A. 1972 Feb;69(2):422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray B. H., Bernlohr R. W. The regulation of aspartokinase in Bacillus licheniformis. Biochim Biophys Acta. 1969 Apr 22;178(2):248–261. doi: 10.1016/0005-2744(69)90394-5. [DOI] [PubMed] [Google Scholar]
- Holzer H., Duntze W. Metabolic regulation by chemical modification of enzymes. Annu Rev Biochem. 1971;40:345–374. doi: 10.1146/annurev.bi.40.070171.002021. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leitzmann C., Bernlohr R. W. Threonine dehydratase of Bacillus licheniformis. II. Regulation during development. Biochim Biophys Acta. 1968 Feb 5;151(2):461–472. doi: 10.1016/0005-2744(68)90114-9. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Martin P., White D., Wong M. C. Changes in activity of glyoxylate cycle enzymes during myxospore development in Myxococcus xanthus. J Bacteriol. 1972 Sep;111(3):784–790. doi: 10.1128/jb.111.3.784-790.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Turnover of intracellular proteins. Annu Rev Microbiol. 1972;26:103–126. doi: 10.1146/annurev.mi.26.100172.000535. [DOI] [PubMed] [Google Scholar]
- Prouty W. F., Goldberg A. L. Effects of protease inhibitors on protein breakdown in Escherichia coli. J Biol Chem. 1972 May 25;247(10):3341–3352. [PubMed] [Google Scholar]
- SUSSMAN M., LOVGREN N. PREFERENTIAL RELEASE OF THE ENZYME UDP-GALACTOSE POLYSACCHARIDE TRANSFERASE DURING CELLULAR DIFFERENTIATION IN THE SLIME MOLD, DICTYOSTELIUM DISCOIDEUM. Exp Cell Res. 1965 Apr;38:97–105. doi: 10.1016/0014-4827(65)90431-3. [DOI] [PubMed] [Google Scholar]
- SUSSMAN M., OSBORN M. J. UDP-GALACTOSE POLYSACCHARIDE TRANSFERASE IN THE CELLULAR SLIME MOLD, DICTYOSTELIUM DISCOIDEUM: APPEARANCE AND DISAPPEARANCE OF ACTIVITY DURING CELL DIFFERENTIATION. Proc Natl Acad Sci U S A. 1964 Jul;52:81–87. doi: 10.1073/pnas.52.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B. M., Stadtman E. R. The regulation of glutamine synthesis in microorganisms. Annu Rev Microbiol. 1970;24:501–524. doi: 10.1146/annurev.mi.24.100170.002441. [DOI] [PubMed] [Google Scholar]
- Smith T. E., Perry M. Escherichia coli phosphoenolpyruvate carboxylase: kinetics and mechanism of inactivation. Arch Biochem Biophys. 1973 Jun;156(2):448–455. doi: 10.1016/0003-9861(73)90293-2. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Kornberg A. Biochemical studies of bacterial sporulation and germination. VI. Origin of spore core and coat proteins. J Biol Chem. 1968 Sep 10;243(17):4588–4599. [PubMed] [Google Scholar]
- Stahly D. P., Bernlohr R. W. Control of aspartokinase during development of Bacillus licheniformis. Biochim Biophys Acta. 1967;146(2):467–476. doi: 10.1016/0005-2744(67)90230-6. [DOI] [PubMed] [Google Scholar]
- Waindle L. M., Switzer R. L. Inactivation of aspartic transcarbamylase in sporulating Bacillus subtilis: demonstration of a requirement for metabolic energy. J Bacteriol. 1973 May;114(2):517–527. doi: 10.1128/jb.114.2.517-527.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


