Abstract
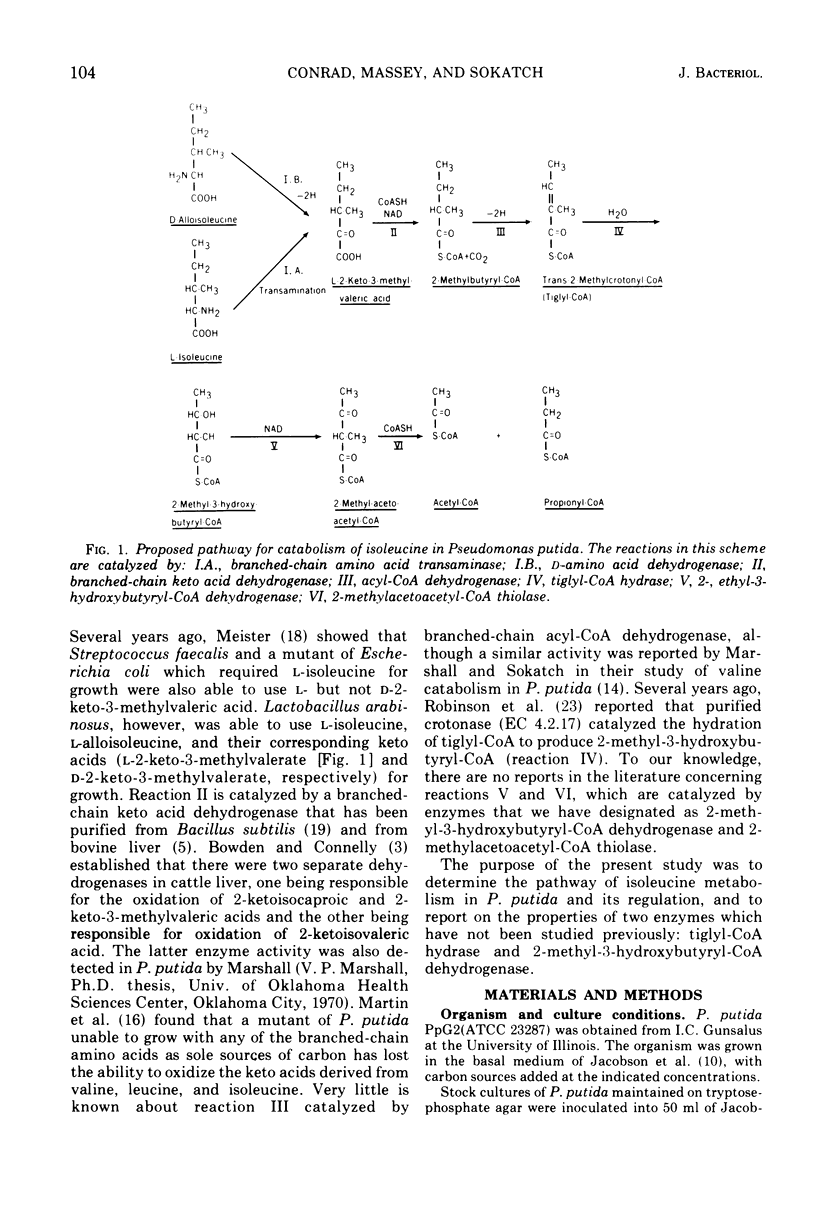
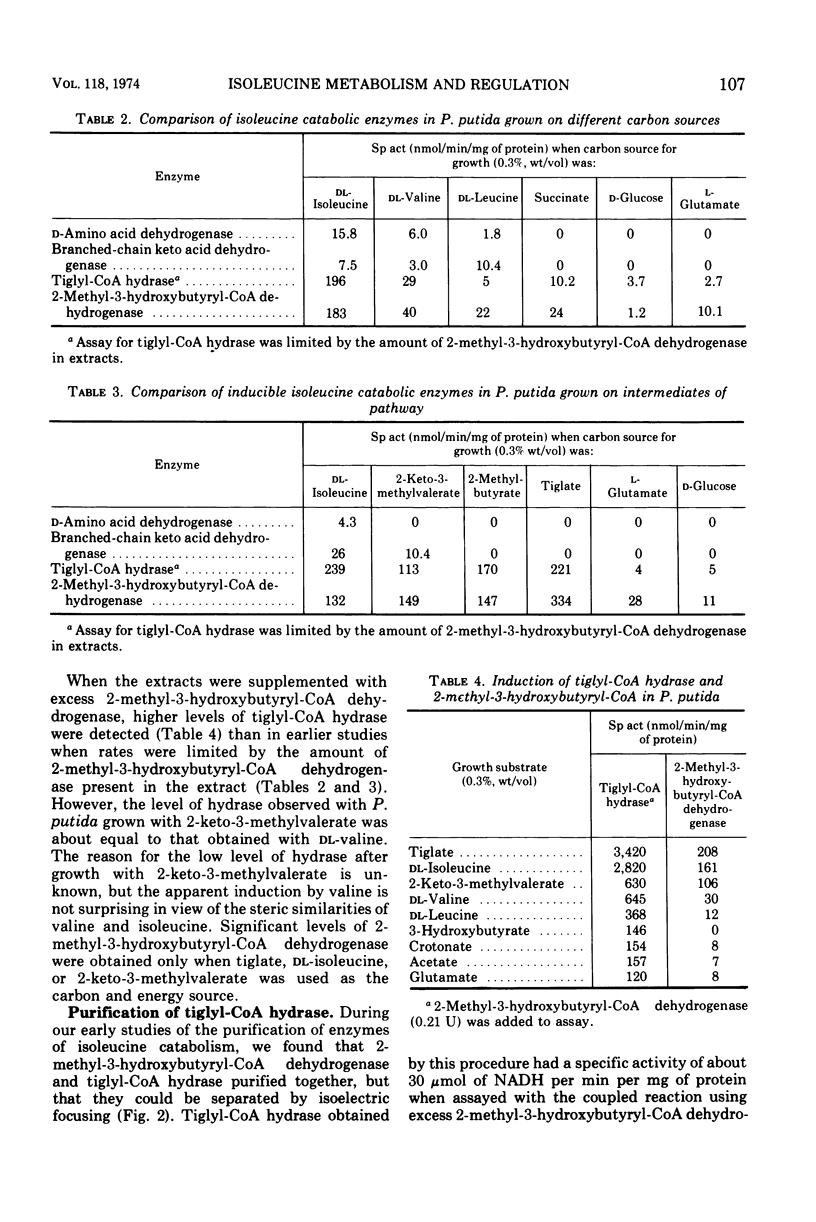
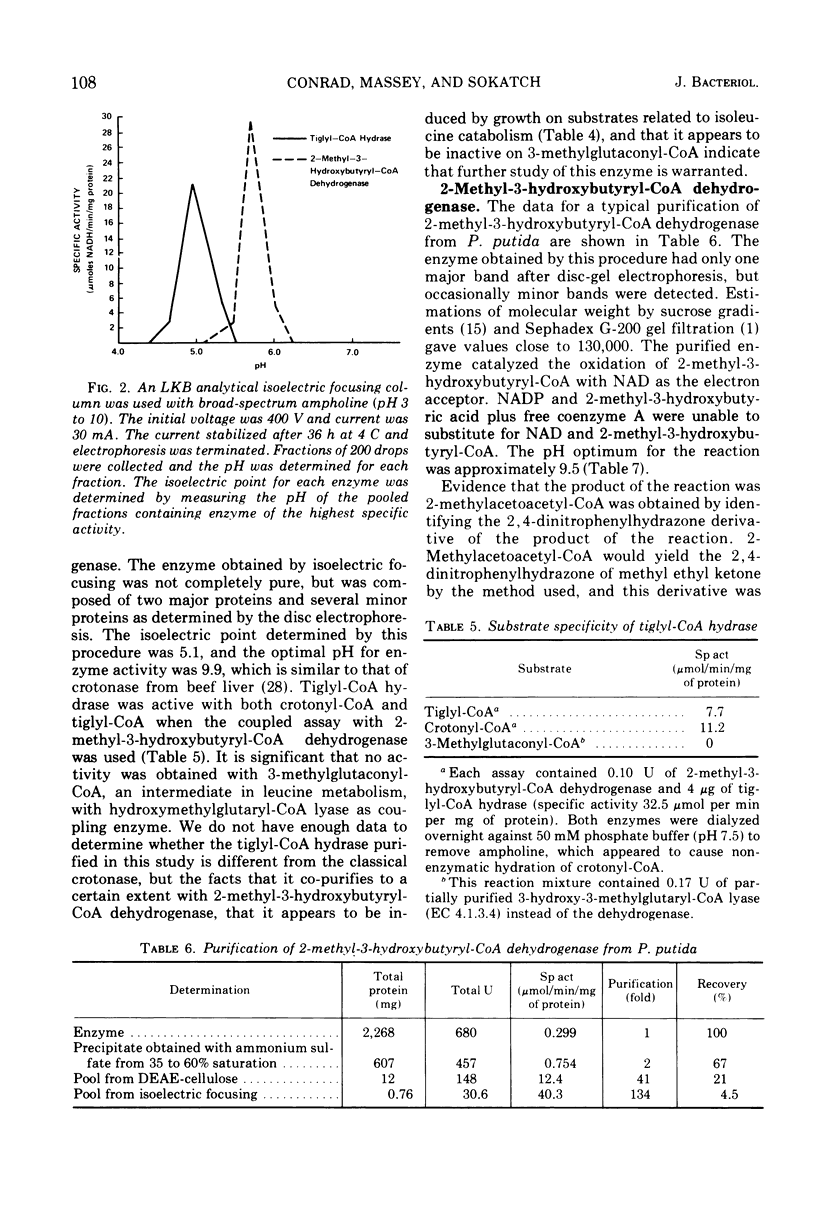
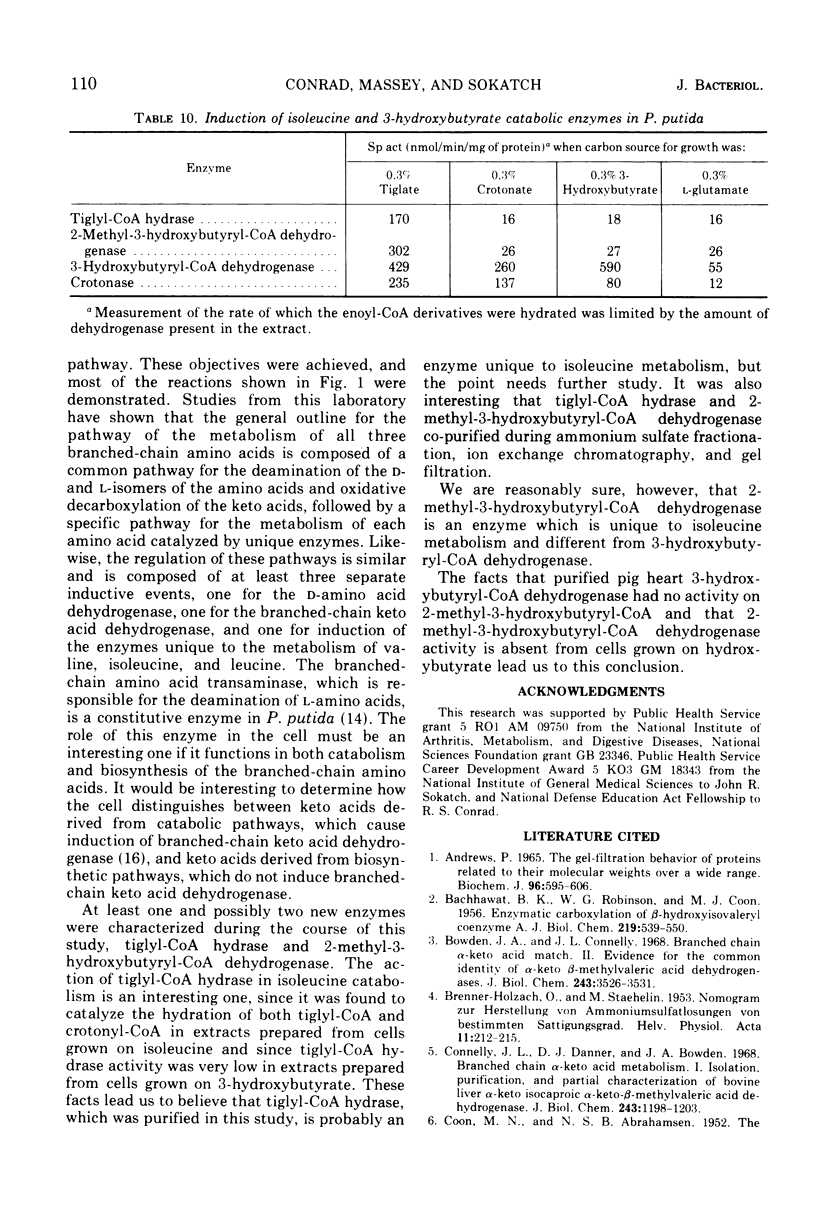
Pseudomonas putida oxidized isoleucine to acetyl-coenzyme A (CoA) and propionyl-CoA by a pathway which involved deamination of d-isoleucine by oxidation and l-isoleucine by transamination, oxidative decarboxylation, and beta oxidation at the ethyl side chain. At least three separate inductive events were required to form all of the enzymes of the pathway: d-amino acid dehydrogenase was induced during growth in the presence of d-isoleucine; branched-chain keto dehydrogenase was induced during growth on 2-keto-3-methylvalerate and enzymes specific for isoleucine metabolism; tiglyl-CoA hydrase and 2-methyl-3-hydroxybutyryl-CoA dehydrogenase were induced by growth on isoleucine, 2-keto-3-methylvalerate, 2-methylbutyrate, or tiglate. Tiglyl-CoA hydrase and 2-methyl-3-hydroxybutyryl-CoA dehydrogenase were purified simultaneously by several enzyme concentration procedures, but were separated by isoelectric focusing. Isoelectric points, pH optima, substrate specificity, and requirements for enzyme action were determined for both enzymes. Evidence was obtained that the dehydrogenase catalyzed the oxidation of 2-methyl-3-hydroxybutyryl-CoA to 2-methylacetoacetyl-CoA. 2-Methyl-3-hydroxybutyryl-CoA dehydrogenase catalyzed the oxidation of 3-hydroxybutyryl-CoA, but l-3-hydroxyacyl-CoA dehydrogenase from pig heart did not catalyze the oxidation of 2-methyl-3-hydroxybutyryl-CoA; therefore, they appeared to be different dehydrogenases. Furthermore, growth on tiglate resulted in the induction of tiglyl-CoA hydrase and 2-methyl-3-hydroxybutyryl-CoA dehydrogenase, but these two enzymes were not induced during growth on crotonate or 3-hydroxybutyrate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACHHAWAT B. K., ROBINSON W. G., COON M. J. Enzymatic carboxylation of beta-hydroxyisovaleryl coenzyme A. J Biol Chem. 1956 Apr;219(2):539–550. [PubMed] [Google Scholar]
- BREMMER-HOLZACH O., STAEHELIN M. Nomogramm zur Herstellung von Ammoniumsulfatlösungen von bestimmtem Sättigunsgrad. Helv Physiol Pharmacol Acta. 1953;11(2):212–215. [PubMed] [Google Scholar]
- Bowden J. A., Connelly J. L. Branched chain alpha-keto acid metabolism. II. Evidence for the common identity of alpha-ketoisocaproic acid and alpha-keto-beta-methyl-valeric acid dehydrogenases. J Biol Chem. 1968 Jun 25;243(12):3526–3531. [PubMed] [Google Scholar]
- COON M. J., ABRAHAMSEN N. S. B., GREENE G. S. The relation of alpha-methyl-butyrate to isoleucine metabolism. II. Propionate formation. J Biol Chem. 1952 Nov;199(1):75–84. [PubMed] [Google Scholar]
- COON M. J., ABRAHAMSEN N. S. B. The relation of alpha-methylbutyrate to isoleucine metabolism. I. Acetoacetate formation. J Biol Chem. 1952 Apr;195(2):805–812. [PubMed] [Google Scholar]
- Connelly J. L., Danner D. J., Bowden J. A. Branched chain alpha-keto acid metabolism. I. Isolation, purification, and partial characterization of bovine liver alpha-ketoisocaproic:alpha-keto-beta-methylvaleric acid dehydrogenase. J Biol Chem. 1968 Mar 25;243(6):1198–1203. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DENTI E., LUBOZ M. P. SEPARATION 2,4-DINITROPHENYLHYDRAZONES OF CARBONYL COMPOUNDS BY THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1965 May;18:325–330. doi: 10.1016/s0021-9673(01)80369-1. [DOI] [PubMed] [Google Scholar]
- Jacobson L. A., Bartholomaus R. C., Gunsalus I. C. Repression of malic enzyme by acetate in Pseudomonas. Biochem Biophys Res Commun. 1966 Sep 22;24(6):955–960. doi: 10.1016/0006-291x(66)90343-3. [DOI] [PubMed] [Google Scholar]
- Li Y. T., Li S. C. Studies on the glycosidases in jack bean meal. II. Sepation of various glycosidases by isoelectric focusing. J Biol Chem. 1968 Jul 25;243(14):3994–3996. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MEISTER A. Utilization and transamination of the stereoisomers and keto analogues of isoleucine. J Biol Chem. 1952 Apr;195(2):813–826. [PubMed] [Google Scholar]
- Marshall V. D., Sokatch J. R. Regulation of valine catabolism in Pseudomonas putida. J Bacteriol. 1972 Jun;110(3):1073–1081. doi: 10.1128/jb.110.3.1073-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall V. P., Sokatch J. R. Oxidation of D-amino acids by a particulate enzyme from Pseudomonas aeruginosa. J Bacteriol. 1968 Apr;95(4):1419–1424. doi: 10.1128/jb.95.4.1419-1424.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. R., Marshall V. D., Sokatch J. R., Unger L. Common enzymes of branched-chain amino acid catabolism in Pseudomonas putida. J Bacteriol. 1973 Jul;115(1):198–204. doi: 10.1128/jb.115.1.198-204.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey L. K., Conrad R. S., Sokatch J. R. Regulation of leucine catabolism in Pseudomonas putida. J Bacteriol. 1974 Apr;118(1):112–120. doi: 10.1128/jb.118.1.112-120.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTON J. E., BULMER G. S., SOKATCH J. R. THE OXIDATION OF D-ALANINE BY CELL MEMBRANES OF PSEUDOMONAS AERUGINOSA. Biochim Biophys Acta. 1963 Oct 8;78:136–147. doi: 10.1016/0006-3002(63)91619-6. [DOI] [PubMed] [Google Scholar]
- Namba Y., Yoshizawa K., Ejima A., Hayashi T., Kaneda T. Coenzyme A- and nicotinamide adenine dinucleotide-dependent branched chain alpha-keto acid dehydrogenase. I. Purification and properties of the enzyme from Bacillus subtilis. J Biol Chem. 1969 Aug 25;244(16):4437–4447. [PubMed] [Google Scholar]
- Norton J. E., Sokatch J. R. Purification and partial characterization of the branched chain amino acid transaminase of Pseudomonas aeruginosa. Biochim Biophys Acta. 1970 May 13;206(2):261–269. doi: 10.1016/0005-2744(70)90109-9. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- ROBINSON W. G., BACHHAWAT B. K., COON M. J. Tiglyl coenzyme A and alpha-methylacetoacetyl coenzyme A, intermediates in the enzymatic degradation of isoleucine. J Biol Chem. 1956 Jan;218(1):391–400. [PubMed] [Google Scholar]
- Stegink L. D., Coon M. J. Stereospecificity and other properties of highly purified beta-hydroxy-beta-methylglutaryl coenzyme A cleavage enzyme from bovine liver. J Biol Chem. 1968 Oct 25;243(20):5272–5279. [PubMed] [Google Scholar]
- Taylor R. T., Jenkins W. T. Leucine aminotransferase. I. Colorimetric assays. J Biol Chem. 1966 Oct 10;241(19):4391–4395. [PubMed] [Google Scholar]


