Abstract
Exposing skin to UVB (280–320 nm) radiation suppresses contact hypersensitivity by a mechanism that involves an alteration in the activity of cutaneous antigen-presenting cells (APC). UV-induced DNA damage appears to be an important molecular trigger for this effect. The specific target cells in the skin that sustain DNA damage relevant to the immunosuppressive effect have yet to be identified. We tested the hypothesis that UV-induced DNA damage in the cutaneous APC was responsible for their impaired ability to present antigen after in vivo UV irradiation. Cutaneous APC were collected from the draining lymph nodes of UVB-irradiated, hapten-sensitized mice and incubated in vitro with liposomes containing a photolyase (Photosomes; Applied Genetics, Freeport, NY), which, upon absorption of photoreactivating light, splits UV-induced cyclobutane pyrimidine dimers. Photosome treatment followed by photoreactivating light reduced the number of dimer-containing APC, restored the in vivo antigen-presenting activity of the draining lymph node cells, and blocked the induction of suppressor T cells. Neither Photosomes nor photoreactivating light alone, nor photoreactivating light given before Photosomes, restored APC activity, and Photosome treatment did not reverse the impairment of APC function when isopsoralen plus UVA (320–400 nm) radiation was used instead of UVB. These controls indicate that the restoration of APC function matched the requirements of Photosome-mediated DNA repair for dimers and post-treatment photoreactivating light. These results provide compelling evidence that it is UV-induced DNA damage in cutaneous APC that leads to reduced immune function.
Exposing skin to UV radiation induces lesions in cellular DNA. Among those lesions, cyclobutane pyrimidine dimers (subsequently referred to as “dimers”) and [6–4] photoproducts predominate and are believed to play an important role in initiating skin cancer (1). UV irradiation of murine skin also reduces certain immune responses, and this suppressed immunity plays a significant role in UV carcinogenesis by allowing highly antigenic, UV-induced skin cancers to grow unimpeded (2, 3). The mechanisms of UVB (280–320 nm)-induced immune suppression are not completely understood; evidence is accumulating, however, that the induction of dimers by UVB represents a key step in the initiation of immune suppression (4–6), although other mechanisms such as the photoisomerization of urocanic acid (7), free-radical formation (8), and signal transduction-mediated activation of transcription factors (9, 10) may play a role as well.
Immunological changes induced by UVB can be manifested locally within the UV-irradiated skin or systemically at sites distant from the UV-irradiated skin, depending on the experimental conditions (11). A well studied, local immunological effect of UV irradiation is inhibition of the contact hypersensitivity (CHS) response, which occurs after a contact sensitizer is applied to UV-irradiated skin of certain strains of mice (reviewed in ref. 12). The CHS response is thought to be initiated mainly by Langerhans cells (13, 14), which are the primary antigen-presenting cells of the epidermis. These cells, and possibly other types of dendritic cells in the skin, migrate after contact sensitization to the draining lymph nodes (15–17), where they form clusters with T lymphocytes (18, 19) and initiate a CHS response. However, UV irradiation of the site before application of a contact sensitizer diminishes the CHS response (20) and induces hapten-specific T suppressor cells (21). Our previous studies provided strong evidence that DNA damage is a molecular trigger for this particular immunosuppressive effect of UVB on CHS (4, 6).
Several studies indicated that cutaneous APC are important targets of UVB in the induction of local immunosuppression. Fluorescein isothiocyanate (FITC)-bearing APC recovered from the draining lymph nodes of epicutaneously sensitized mice induced CHS when injected into the footpads of syngeneic recipients; such APC recovered from mice sensitized with FITC through UVB-irradiated skin had a reduced ability to induce CHS (22, 23). In addition, in vitro exposure of Langerhans cells to UVB impaired their ability to stimulate proliferation of T helper cell-1 (Th-1)-type T cell clones (24), and the interaction produced a state of anergy in the Th-1 cells (25). Because cutaneous APC are accessible to the direct, DNA-damaging effects of UVB (26), and because antigen presentation is a critical step in determining the outcome of the immune response, we hypothesized that the formation of UVB-induced dimers in the DNA of cutaneous APC was responsible for their altered function in UV-irradiated murine skin (27).
Our recent study (27) supported this hypothesis by showing that APC in both the dermis and the epidermis of UV-irradiated mice contained dimers, and that dendritic cells with DNA damage persisted in the skin for several days. Upon epicutaneous sensitization with FITC 3 days after UV, dimers-containing, FITC-bearing dendritic cells migrated to the draining lymph nodes; this draining lymph node cell population had impaired APC function, as indicated by their reduced ability to induce CHS in vivo. Topical application of liposome-encapsulated T4 endonuclease V to the UV-irradiated skin, which increases the rate of DNA excision repair, reduced the number of dimer+ cells in the draining lymph nodes. Concomitantly, it restored the APC activity of the draining lymph node cells and prevented the induction of suppressor T lymphocytes (Ts). Although these studies showed a correlation between UV-induced DNA damage in APC and impaired APC function, they did not rule out the possibility that cytokine production by UV-irradiated keratinocytes was responsible for the effects on cutaneous APC function. UV irradiation of keratinocytes has been demonstrated to induce the production of cytokines that can down-regulate APC function (28), and recent evidence also implicates DNA damage as a trigger for cytokine production (29). Because liposome-encapsulated T4 endonuclease V was shown to be taken up by both keratinocytes and epidermal Langerhans cells in murine skin in vivo (30), they reduced DNA damage in both cell types (27, 29). Thus, it was unclear whether the effect of UVB on the activity of cutaneous APC resulted from DNA damage in the APC or DNA damage in keratinocytes, which secondarily affected APC function by altering the cytokine profile of neighboring keratinocytes.
To resolve this issue, we devised an approach that enabled us to reduce DNA damage in the APC without affecting keratinocytes. We used a liposome-encapsulated dimer-specific photolyase from Anacystis nidulans (Photosomes, Applied Genetics, Freeport, NY) to treat dimer-containing draining lymph node cells in vitro. This DNA repair enzyme (31) binds to dimers in cells and upon absorption of photoreactivating light splits the dimers to pyrimidine monomers (32). In these experiments, APC were isolated from the draining lymph nodes of UVB-irradiated, FITC-sensitized mice and treated with Photosomes and photoreactivating light in vitro. This treatment permitted the instantaneous reversal of dimer formation in the draining lymph node cells in the absence of keratinocytes, so that APC repair could be separated from secondary effects of keratinocyte repair on APC function. This approach has the additional advantage over the use of liposome-encapsulated T4 endonuclease V in that it does not require the APC, which have low nucleotide pools, to perform DNA repair synthesis. We used this approach to determine whether reducing the amount of UV-induced DNA damage in APC would affect their ability to induce CHS and Ts in vivo and interferon (IFN)-γ production by T cells in vitro. By testing the effects of enhanced repair using an enzyme that acts by a completely different mechanism than T4 endonuclease V, we also could examine whether restoration of immune competence was restricted to a certain type of enzymatic repair or related to the removal of dimers.
MATERIALS AND METHODS
Mice.
Specific pathogen-free C3H/HeN (mammary tumor virus- negative) female mice were obtained from the Frederick Cancer Research Center Animal Production Area (Frederick, MD). Specific pathogen-free C3H SCID mice were originally obtained from Harlan Sprague–Dawley and were propagated in the M.D. Anderson Cancer Center facility. Age-matched mice from 10 to 12 weeks old were used in these experiments. They were housed in filter-protected cages, and ambient lighting was controlled to provide 12 h light/12 h dark cycles. Autoclaved National Institutes of Health open-formula mouse chow and water were provided ad libitum. The animal facility is accredited by the American Association for Accreditation of Laboratory Animal Care, and all animal protocols were approved by the Institutional Animal Care and Use Committee.
UVB Irradiation.
The UVB source was a bank of six FS40 sunlamps (National Biological, Twinsburg, OH), which emit approximately 60% of their radiation within the UVB range and have a peak emission at 313 nm. The average irradiance 20 cm below the source was approximately 9 W/m2, as measured by an IL-700 radiometer (International Light, Newburyport, MA). The average irradiance at the level of the animals at this distance was approximately 4.5 W/m2 because of screening by the cage lids. Before irradiation, the animals’ dorsal fur was shaved with electric clippers. Unless otherwise indicated, the mice received 5.0 kJ/m2 of UVB, which is approximately twice the minimum erythemal dose for this strain.
Photoreactivating Light.
Two fluorescent black-light bulbs (F15T8, Sylvania Electric Products, Fall River, MA) were used as a source of photoreactivating light for in vitro irradiation of Photosome-treated APC. The light was filtered through an 0.05-mm sheet of Mylar to eliminate radiation below 320 nm. About 99% of the radiation emitted was within the UVA (320–400 nm) range, as determined by an Optronix 742 Spectroradiometer (Optronix Laboratories, Orlando, FL); peak emission occurred at 366 nm. The irradiance was 22 W/m2, at a distance of 20 cm.
UVA Irradiation.
The source used to UVA-irradiate isopsoralen-treated mice was a Dermalight 2001 with an optical H1 filter (Dermalight Systems, Studio City, CA). The spectral irradiance was measured by an Optronics 742 spectroradiometer. Of the radiation received, 99.5% was within the UVA range and had a peak emission at 366 nm. The energy output was measured with an IL-700A research radiometer with a UVA detector. The average irradiance was 99 W/m2.
Isopsoralen plus UVA (IPUVA) Treatment.
Isopsoralen (angelicin), obtained as a crystal powder from HRI Associates (Emeryville, CA), was dissolved in 70% alcohol to form a 0.214% (wt/vol) solution. The drug was applied topically in a 100-μl volume on the shaved dorsal skin of the mice; 45–60 min thereafter, the mice were irradiated with 25 kJ/m2 of UVA radiation (Dermalight 2001). This treatment was given three times during one week, every other day (33).
Photosomes.
Dimer-specific photolyase-containing liposomes (Photosomes) were prepared by encapsulating Anacystis nidulans photolyase in liposomes composed of phosphatidylcholine, phosphatidylethanolamine, oleic acid, cholesterol hemisuccinate (2:2:1:5 molar ratio) by the detergent dialysis method (34). The concentration of the entrapped enzyme was determined by ELISA (34) using a rabbit polyclonal antibody raised against the purified photolyase, and is expressed as micrograms of encapsulated enzyme per milliliter of vehicle. The encapsulated activity was measured by determining the removal of T4 endonuclease V-sensitive sites from UV-irradiated λ DNA (35).
Contact Sensitization.
Mice were sensitized 3 days after UVB irradiation or immediately after the third IPUVA treatment on the shaved, dorsal skin by application of 400 μl of a solution of 0.5% FITC (isomer I, Aldrich) in acetone-dibutylphthalate (1:1, vol/vol). Six days later, ear thickness was measured, and the mice were challenged by an application of 5 μl of 0.5% FITC to the inner and outer surfaces of each ear. Ear swelling was measured with a spring-loaded micrometer 24 h later (27). Each experimental group contained at least five mice.
Cell Suspensions.
Single-cell suspensions of draining lymph nodes were made 18 h after FITC sensitization, as described previously (27), and suspended in RPMI medium 1640 (GIBCO) with 5% fetal bovine serum (HyClone). The cells were layered under 3 ml of 18% metrizamide (Sigma) and centrifuged at 1,000 × g for 10 min at 4°C. The dendritic cell-enriched interface cells were collected and washed three times with RPMI medium 1640/5% fetal bovine serum.
Photoreactivation.
The metrizamide-purified cells were resuspended in RPMI medium 1640/4% fetal bovine serum and incubated with Photosomes (30 μg/ml) for 60 min at 37°C in the dark. The cells were washed once, resuspended in PBS, and exposed in a Petri dish as a monolayer to photoreactivating light (5.0 kJ/m2 UVA). As controls, cells were treated with Photosomes or photoreactivating light alone, or they were first exposed to photoreactivating light and then incubated with Photosomes in the dark.
Draining Lymph Node Cell Transfer.
To test the in vivo antigen-presenting activity of the metrizamide-purified draining lymph node cells, 3 × 104 FITC+ cells in 50 μl of a cell suspension were injected into the hind footpads of normal, syngeneic mice (27). Cells from the same cell suspension also were collected on slides by cytospin centrifugation for immunohistochemical analysis.
Spleen Cell Transfer.
To test for the presence of splenic suppressor cells, spleen cell suspensions were prepared in RPMI medium 1640 from mice immunized by injection of draining lymph node cells from FITC-sensitized mice (23). Five-tenths milliliters of these cells were injected i.v. into normal, syngeneic recipient mice at a concentration of 2 × 108 viable, nucleated cells per ml. Recipient mice were sensitized immediately on shaved dorsal skin with FITC and challenged on the ears 6 days later, as described above (23).
Immunohistochemical Staining for Dimers.
Cells containing dimers were detected using the mouse mAb specific for dimers developed by Roza et al. (36). It is estimated that this antibody can detect cells with four or more dimers per megabase of DNA. A horseradish peroxidase-conjugated goat anti-mouse antibody (Boehringer Mannheim) was used to visualize the dimer-specific antibody (27). The dimer+ cells were easily distinguished by the red-brown coloration confined to the cell (27) and were proportional to UVB dose; a dose of 1 kJ/m2 UBV produced barely detectable dimer+ cells in C3H mouse skin (27). The percentage of dimer+ cells in the pooled lymph nodes from at least 15 mice was determined by counting a minimum of 200 cells in each of five fields under a Nikon Optiphot microscope (Nikon, Garden City, NY). Controls included frozen skin sections from UV-irradiated and unirradiated mice and samples stained with only the second antibody.
In Vitro Assay of IFN-γ.
Primed T cells were obtained by nylon wool purification (37) from the draining lymph nodes of mice sensitized epicutaneously with FITC 5 days earlier. One hundred thousand dendritic cells were cultured with 2 × 105 T cells in a volume of 200 μl/well in RPMI medium 1640/10% fetal bovine serum at 37°C in 5% CO2/95% air for 39 h. Supernatants were collected and IFN-γ was measured by ELISA (PharMingen; sensitivity 10 units/ml).
Statistical Analysis.
The significance of differences between experimental groups was determined by Student’s two-tailed t test. A difference was considered to be significant when the probability of no difference was ≤0.05.
RESULTS
Effect of Photosomes on Percentage of Dimer+ Draining Lymph Node Cells.
We determined whether in vitro treatment with Photosomes and photoreactivating light would split dimers in DNA and thereby reduce the percentage of dimer+ cutaneous APC derived from the draining lymph nodes of UV-irradiated mice. Mice were exposed to 5 kJ/m2 of UVB on dorsal skin, a dose sufficient to produce immunohistochemically detectable DNA damage in APC. Three days later, they were sensitized through the irradiated skin with FITC; 18 h later, the draining lymph node cells were collected and enriched for dendritic cells on a metrizamide gradient. The resulting cell population, which generally contained 75–90% dendritic cells, was incubated with Photosomes for 1 h, washed extensively, and then exposed to 5 kJ/m2 photoreactivating light. Cytospin preparations of the cells were stained by immunoperoxidase for the presence of dimers. In these experiments the antibody staining measures DNA damage remaining in a few UV irradiated cells amid many more unirradiated cells in the draining lymph node. We follow the frequency of the most heavily damaged cells, the dimer+ cells detected by immunohistochemistry, as a marker for repair in all the UV-irradiated cells.
Dendritic cell preparations obtained from UV-irradiated mice contained 19 ± 2 dimer+ cells/103 metrizamide-purified dendritic cells. The experiments presented in Table 1 show that the Photosome/photoreactivating light treatment significantly reduced the fraction of dimer+ cells in the draining lymph nodes of UV-irradiated mice, whereas treatment with either Photosomes or photoreactivating light alone had no significant effect. Overall, the reduction in the percentage of dimer+ cells by Photosome/photoreactivating light treatment was 53 ± 17%. A lower photoreactivating light dose of 1 kJ/m2 UVA produced a reduction in the fraction of dimer+ cells of 12% (data not shown). None of the treatments affected the viability of the cells significantly, as determined by their ability to exclude trypan blue.
Table 1.
Effect of Photosome/photoreactivating light treatment on number and activity of dimer+ draining lymph node (DLN) cells
| Treatment of DLN cells*
|
No. of dimer+ cells per 103 DLN cells (% reduction)†
|
CHS response from exp. 2 (ear swelling)
|
|||||
|---|---|---|---|---|---|---|---|
|
In vivo
|
In vitro
|
||||||
| UVB | FITC | Photosomes | PRL | Exp. 1 | Exp. 2 | mm × 10−2 | % reduction‡ |
| + | + | − | − | 20 ± 2 | 18 ± 2 | 3.8 ± 0.6 | 71 |
| + | + | + | − | 19 ± 2 (5) | 14 ± 2 (22) | 3.7 ± 0.4 | 73 |
| + | + | − | + | 17 ± 2 (15) | 16 ± 3 (11) | 4.5 ± 0.6 | 57 |
| + | + | + | + | 10§ ± 2 (50) | 6§ ± 1 (67) | 7.8§ ± 0.7 | 0 |
| − | + | − | − | 0 | 0 | 7.4§ ± 0.8 | — |
| − | + | + | + | 0 | 0 | 7.5§ ± 0.5 | 0 |
| − | − | − | − | — | — | 2.3 ± 0.5 | — |
DLN cells were collected 18 h after sensitization with FITC, pooled, and enriched for dendritic cells on metrizamide gradients. UVB-irradiated donors received 5 kJ/m2 on shaved dorsal skin 3 days before FITC. DLN cells from 15 (Exp. 1) or 75 (Exp. 2) UVB+FITC-treated mice were pooled and separated into four aliquots after metrizamide purification for photolyase/photoreactivating light (PRL) treatment. Cytospin preparations were made from cells of each treatment group. In Exp. 2, the remaining cells were injected into recipient mice for induction of CHS. The number of dimer+ cells was determined by counting six fields containing 200–600 cells each. The results (mean ± SEM) are representative of multiple independent experiments.
Versus UVB+FITC group (first line).
Versus FITC group (positive control, fifth line), corrected for background swelling in untreated animals (seventh line).
P < 0.05 versus UVB+FITC group (first line).
Effect of Photosomes on Antigen-Presenting Activity of Draining Lymph Node Cells.
To investigate the antigen-presenting activity of the dendritic draining lymph node cells after repair of dimers by photoreactivation, metrizamide-purified draining lymph node cells from UV-irradiated or unirradiated, FITC-sensitized mice were injected into the hind footpads of syngeneic recipient mice. The CHS response that developed in the recipients was used as a measure of the antigen-presenting activity of the draining lymph node cells injected (17, 22, 23). The activity of the draining lymph node cell preparations from experiment 2 is shown in Table 1. As demonstrated in our previous studies, the ability of the draining lymph node cells to induce CHS in vivo is markedly impaired by previous exposure of the skin to UVB radiation, even though the number of FITC+ APC in the population is not diminished (22, 23). Treatment of these cells in vitro with Photosomes plus photoreactivating light, but not with either one alone, restored their ability to induce CHS in recipient mice.
A characteristic of the immunohistochemical technique is that the intensity of dimer+ staining can vary among experiments. Likewise, the magnitude of the CHS response can differ because of variability in the animals and reagents. This is addressed by including both positive and negative controls in each experiment. For example, the effect was not caused by a nonspecific stimulatory effect of the Photosome/photoreactivating light treatment on APC activity because there was no enhanced activity of draining lymph node cells from unirradiated mice treated in vitro with Photosomes and photoreactivating light.
We investigated the effect of Photosome/photoreactivating light treatment on the induction of Ts by testing the recipients of draining lymph node cells for the ability of their spleen cells to suppress the induction of CHS in secondary hosts. Draining lymph node cell donors were treated as described above to measure CHS. Immediately after the ear swelling response was measured, the animals’ spleens were removed, and spleen cell suspensions were injected i.v. into normal mice, which were immediately sensitized epicutaneously with FITC. In this way, we measured the ability of the draining lymph node recipients’ spleen cells to suppress the induction of a primary CHS.
The results of such an experiment are illustrated in Tables 2 and 3. Table 2 shows the percentage of dimer+ draining lymph node cells from UV-irradiated mice with and without Photosome/photoreactivating light treatment and their ability to induce CHS. The initial percentage of dimer+ cells was lower in this particular experiment than in the experiments in Table 1. As before, however, treatment with Photosomes before photoreactivating light (Group 3) reduced the number and restored the CHS-inducing activity of the dimer+ cells, whereas treatment of the cells with photoreactivating light before Photosomes (Group 2) had no effect on either parameter. Table 3 shows the results of the spleen cell transfer, in which draining lymph node cells from UV-irradiated mice induced transferable suppression of the CHS response (Group 1). In vitro photoreactivation of the draining lymph node cell population eliminated their ability to induce suppressor cells in the recipient mice (Group 3), whereas pretreatment with photoreactivating light had no effect (Group 2).
Table 2.
Effect of Photosome/photoreactivating light (PRL) treatment on suppressor cell induction: Draining lymph node (DLN) Cell Transfer
| Group | Treatment of DLN cells*
|
No. of dimer+ cells per 103 DLN (% reduction)† | CHS response in DLN recipients (ear swelling)
|
||||
|---|---|---|---|---|---|---|---|
|
In vivo
|
In vitro
|
||||||
| UVB | FITC | Photosomes/PRL | PRL/Photosomes | mm × 10−2 | % reduction‡ | ||
| 1 | + | + | − | − | 10 ± 1 | 3.3 ± 0.6 | 46 |
| 2 | + | + | − | + | 10 ± 0 (0) | 2.1 ± 0.4 | 67 |
| 3 | + | + | + | − | 7§ ± 1 (30) | 5.9§ ± 0.7 | 0 |
| 4 | − | + | − | − | 0 | 5.9§ ± 0.5 | — |
| 5 | − | + | + | − | 0 | 5.9§ ± 0.5 | 0 |
| 6 | No cells, challenge only | — | 0.2 ± 0.1 | — | |||
DLN cells from 20 normal and 40 UV-irradiated mice were collected 18 h after FITC sensitization, pooled, and enriched for dendritic cells. Results are presented as mean ± SEM.
Versus UVB+FITC group; the number of dimer+ cells was counted in six fields containing 200–600 cells each.
Versus FITC group (positive control).
P ≤ 0.05 versus UVB+FITC group.
Table 3.
Effect of Photosome/photoreactivating light treatment on suppressor cell induction: Spleen cell transfer
| Treatment group | CHS response in spleen cell recipients (ear swelling)
|
|
|---|---|---|
| mm × 10−2* | % reduction† | |
| Spleen cell donor groups‡ | ||
| Group 1 | 3.3 ± 0.5 | 50 |
| Group 2 | 3.3 ± 0.4 | 50 |
| Group 3 | 7.9§ ± 0.8 | 0 |
| Group 4 | 7.2§ ± 0.8 | 0 |
| Group 5 | 7.1§ ± 0.5 | 0 |
| FITC only (positive control) | 6.3§ ± 0.4 | — |
| Challenge only (negative control) | 0.2 ± 0.1 | — |
Mean ± SEM of five mice.
Versus FITC group (positive control).
Spleen cell donors are mice immunized with draining lymph node cells from indicated treatment groups of Table 2.
P ≤ 0.05 versus UVB+FITC group (Group 1).
Specificity of Photosome/Photoreactivating Light Treatment for UVB-Induced Immune Suppression.
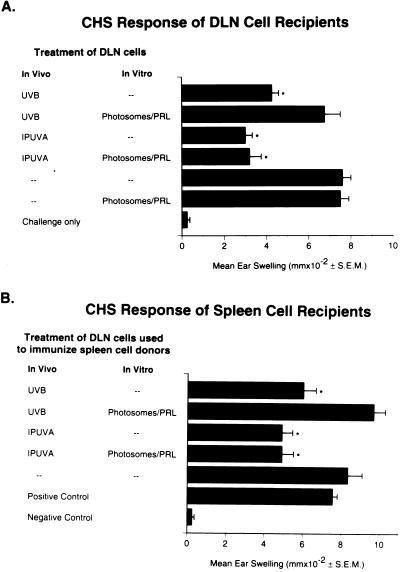
As a control for the specificity of the Photosome/photoreactivating light treatment in reversing dimers, mice were treated in vivo with IPUVA. This treatment causes DNA damage by inducing monofunctional psoralen adducts in DNA, rather than dimers. Based on preliminary dose-response studies (not shown), a regimen of IPUVA was selected to produce impaired APC activity comparable to that produced by UVB irradiation. Fig. 1A shows that draining lymph node cells from IPUVA-treated, FITC-sensitized mice induced a diminished CHS response in syngeneic recipients compared with draining lymph node cells from mice given only FITC; the reduction was similar to that induced by draining lymph node cells from UVB-irradiated mice. Although in vitro Photosome/photoreactivating light treatment of the draining lymph node cells restored the activity of APC from UVB-irradiated mice, this treatment had no effect on the activity of draining lymph node cells from IPUVA-treated mice. Furthermore, transfer of spleen cells from the draining lymph node cell recipients demonstrated that suppressor cells were induced by IPUVA treatment of the draining lymph node cell donors and that this activity was also unaffected by Photosome/photoreactivating light treatment (Fig. 1B).
Figure 1.
Effect of Photosomes/photoreactivating light treatment on the activity of draining lymph node cells from UVB-irradiated or IPUVA-treated mice. Draining lymph node cells from FITC-sensitized donors were incubated in vitro with Photosomes for 1 h and exposed to photoreactivating light. After photoreactivation, the cells were injected into the footpads of normal mice to assess their ability to induce CHS and Ts. ∗, P ≤ 0.05 versus positive control. (A) CHS response of mice sensitized with draining lymph node cells obtained from mice given FITC through UVB-irradiated or IPUVA-treated skin. (B) Ability of spleen cells from recipient mice in A to suppress the induction of CHS to FITC in secondary recipients.
Effect of Photosomes on IFN-γ Production by T Cells.
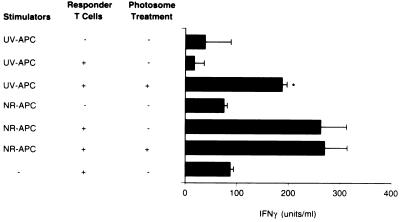
Several lines of evidence suggest that UV irradiation, both in vivo (38, 39) and in vitro (24, 25), interferes with the ability of APC to trigger Th-1 cells. To investigate whether dimer removal from UV-damaged APC affected their ability to activate a Th-1-type response, we assessed the effect of photoreactivation on stimulation of IFN-γ production by FITC-primed T cells. As before, draining lymph node cells were isolated from mice treated in vivo with UVB and FITC, and the dendritic cells were treated in vitro with Photosomes and photoreactivating light. The draining lymph node cells were then cocultured with T cells obtained from the draining lymph nodes of FITC-primed mice, and the supernatants were assayed for the Th-1 cytokine IFN-γ (Fig. 2). T cells produced IFN-γ in response to draining lymph node cells from normal mice; Photosome/photoreactivating light treatment did not affect this activity. The response dropped to background levels when T cells were cocultured with draining lymph node cells from UVB-irradiated mice. Treatment of the latter cell population with Photosomes and photoreactivating light restored the response to 83 ± 18% of the control levels (average of three experiments).
Figure 2.
IFN-γ production by FITC-primed T cells after stimulation with APC from UV-irradiated FITC-sensitized mice (UV-APC) or nonirradiated, FITC-sensitized mice (NR-APC). IFN-γ production was measured by ELISA after 39 h of culture. Metrizamide-purified draining lymph node dendritic cells (1 × 105) were cultured with 2 × 105 lymph node T cells from FITC-primed mice. Draining lymph node cells were treated in vitro with Photosomes, followed by photoreactivating light. ∗, P ≤ 0.001.
DISCUSSION
We are investigating the role and cellular target of UV-induced DNA damage in the impaired induction of CHS in UV-irradiated mice. In this local model of immune suppression, UV irradiation alters the induction phase of the CHS response and leads to production of hapten-specific Ts (20–23). These manifestations of UV irradiation suggested that antigen presentation, particularly presentation to naive T cells, could be the locus of the immune response perturbed by UV irradiation. In these studies, we focused on the role of DNA damage in APC and asked specifically whether dimers in cutaneous APC themselves are responsible for their altered function. Our approach involved collecting dimer-containing APC from the lymph nodes of FITC-sensitized mice and subjecting these cells to photorepair in vitro. Although this approach permits only the detection of cells containing >4 dimers per megabase of DNA (36), removal of DNA damage should reduce the number of detectable dimer+ cells below the limit of detection, thus providing a surrogate marker for the actual number of dimer+ cells. We reasoned that if DNA damage in these cells was responsible for their altered function, then repairing the damage in vitro, in the absence of other cutaneous target cells such as keratinocytes, should restore their APC activity. This was indeed the case; in vitro reversal of dimers in the dendritic cells derived from the draining lymph nodes of in vivo UV-irradiated, FITC-sensitized mice completely restored their ability to induce CHS and trigger production of IFN-γ from FITC-primed T cells. This result implies that keratinocyte-derived cytokines alone are not responsible for down-regulating the activity of these cutaneous APC after UV-irradiation.
Dimers were causally related to the restoration of APC activity because Photosome/photoreactivating light treatment failed to increase the activity of APC from either unirradiated mice or mice immunosuppressed with isopsoralen plus UVA radiation, and photoreactivating light before Photosome treatment likewise had no effect. This ruled out the possibility of a nonspecific stimulatory effect of the Photosome treatment on APC function. It also demonstrated that the effect of the photolyase on APC activity matched its specificity for UVB damaged DNA, because IPUVA treatment produces monofunctional psoralen adducts in DNA, rather than dimers. Isopsoralen, rather than the more common 8-methoxypsoralen, was used for these experiments to circumvent the potential complication of interstrand crosslinks formation in DNA during the in vitro photoreactivating light treatment. This might have occurred with 8-methoxypsoralen, but it could not occur with isopsoralen, which only forms monoadducts in DNA. Notably, agents that induce either monofunctional adducts or DNA crosslinks also produce local suppression of CHS (32). This implies that DNA damage in general causes immune suppression, which may be part of a global cellular response to genetic injury. This is not to say that DNA damage is the only cause, but it is clearly an important cause of UV-induced suppression of the immune response.
That the Photosome/photoreactivating light treatment reduced DNA damage in the APC was evident from experiments in which dimers were detected by an antibody specific for these lesions. The number of dimer+ cells was reduced by approximately 50% after the in vitro Photosome/photoreactivating light treatment described here, similar to the reduction after applying liposome-encapsulated T4 endonuclease V to UV-irradiated murine skin in vivo (27). As we have noted before (5, 29), this amount of DNA repair is sufficient to restore immunologic reactivity completely in several systems of photoimmune suppression. Not all dimers must be repaired before complete immune function is restored, perhaps because in mouse cells not all dimers are of equal biological importance. For example, DNA damage in inactive genes may be irrelevant for biological function; alternatively, a threshold number of dimers may be required to inhibit function, and repairing a small number of dimers with Photosome/photoreactivating light treatment may reduce their number below the threshold.
The most interesting question raised by these studies, however, is how persistent dimers in dendritic cells impaired or altered their APC function. The dimer+ APC that remain in the skin at 3 days after irradiation obviously have not sustained sufficient DNA damage to cause cell death, but they may be functionally arrested, perhaps because of repair-induced depletion of their already low deoxyribonucleotide pools (40). In this state, the cells may have a reduced level of transcription of the genes required for effective antigen presentation, such as intercellular adhesion molecule-1, B7, major histocompatibilty complex class II, interleukin 1, or interleukin 12, leading to their decreased expression. Photosome/photoreactivating light treatment reverses dimers without introducing excision repair patches, and in this manner may release the APC from the arrested state.
In addition, a single dimer located in a transcribed strand of DNA can block transcript elongation by RNA polymerase II (41), and Photosome/photoreactivating light treatment may selectively remove dimers from actively transcribed genes. If this were true it means that a very small number of dimers can block transcription of genes essential to APC function, because a 5 kJ/m2 dose of UVB produces about one dimer per 104 bases (35). As a consequence, this implies that the number of potential target genes participating in antigen processing and/or presentation is large.
A final possibility is that the presence of a threshold level of DNA damage anywhere in the genome of an APC may activate a cascade of cytokine and growth regulatory genes. UV irradiation of many types of cells is known to induce the expression of sets of genes involved in DNA repair and restoration of cellular function (42). We have shown that, in murine keratinocytes in vitro, UV-induced DNA damage activates interleukin 10 production (29), which down-regulates immune function. Similarly, UV irradiation of cutaneous APC may induce the production of interleukin 10, which could serve as an autocrine inhibitor of antigen-presenting cell function and a paracrine inhibitor of unirradiated APC (28). Photosome/photoreactivating light treatment may remove a sufficient amount of DNA damage to abrogate the cytokine signal.
Regardless of the mechanism, our results established a causal relationship between the presence of persistent dimers in cutaneous APC and their altered immune function. This altered APC activity does not seem to be secondary to cytokines produced by UV-irradiated keratinocytes; however, such cytokines may be needed for Ts induction or may contribute in other ways to the local immunosuppressive milieu of UV-irradiated skin.
Acknowledgments
We appreciate the assistance of Patricia Cox and Adrienne O’Connor in performing these studies. A.A.V. and A.M.M. are fellows of the Dermatology Foundation (Reed & Carnrick Pharmaceutical and Dermatik Research Fellowships, respectively). This research was supported by National Institutes of Health Grants RO1 CA52457, RO1 ES07327, and CA-16672.
ABBREVIATIONS
- APC
antigen-presenting cell(s)
- CHS
contact hypersensitivity
- FITC
fluorescein isothiocyanate
- IPUVA
isopsoralen plus UVA
- Ts
suppressor T lymphocyte(s)
- Th
T helper cell(s)
- UVA
320–400 nm ultraviolet radiation
- UVB
280–320 nm ultraviolet radiation
- IFN
interferon
References
- 1.Brash D E, Rudolph J A, Simon J A, Lin A, McKenna G J, Baden H P, Halperin A J, Ponten J. Proc Natl Acad Sci USA. 1991;88:10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kripke M L, Fisher M S. J Natl Cancer Inst. 1976;57:211–215. doi: 10.1093/jnci/57.1.211. [DOI] [PubMed] [Google Scholar]
- 3.Fisher M S, Kripke M L. Science. 1982;216:1133–1134. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- 4.Applegate L A, Ley R D, Alcalay J, Kripke M L. J Exp Med. 1989;117:1117–1131. doi: 10.1084/jem.170.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kripke M L, Cox P A, Alas L G, Yarosh D B. Proc Natl Acad Sci USA. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kripke M L, Cox P A, Bucana C, Vink A A, Alas L, Yarosh D B. Exp Dermatol. 1996;5:173–180. doi: 10.1111/j.1600-0625.1996.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 7.Noonan F P, DeFabo E C. Immunol Today. 1992;13:250–254. doi: 10.1016/0167-5699(92)90005-R. [DOI] [PubMed] [Google Scholar]
- 8.Caceres-Dittmar G, Ariizumia K, Xu S, Tapia F J, Bergstresser P R, Takashima A. Photochem Photobiol. 1995;62:176–183. doi: 10.1111/j.1751-1097.1995.tb05255.x. [DOI] [PubMed] [Google Scholar]
- 9.Devary Y, Rosette C, DiDonata J A, Karin M. Science. 1993;261:442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- 10.Simon M M, Aragane Y, Schwartz A, Luger T A, Schwarz T. J Invest Dermatol. 1994;102:422–427. doi: 10.1111/1523-1747.ep12372194. [DOI] [PubMed] [Google Scholar]
- 11.Kripke M L. Immunol Rev. 1984;80:87–102. doi: 10.1111/j.1600-065x.1984.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 12.Streilein J W, Taylor J R, Vincek V, Kurimoto I, Schimizu T C, Golomb C. Immunol Today. 1994;15:174–179. doi: 10.1016/0167-5699(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 13.Tamaki K, Fujiwara H, Katz S I. J Invest Dermatol. 1981;76:275–278. doi: 10.1111/1523-1747.ep12526115. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan S, Bergstresser P R, Streilein J W. J Invest Dermatol. 1985;84:249–252. doi: 10.1111/1523-1747.ep12265316. [DOI] [PubMed] [Google Scholar]
- 15.Macatonia S E, Edwards A J, Knight S C. Immunology. 1986;59:509–514. [PMC free article] [PubMed] [Google Scholar]
- 16.Macatonia S E, Edwards A J, Griffiths S, Fryer P R. J Exp Med. 1987;166:1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kripke M L, Munn C J, Jeevan A, Tang J-M, Bucana C. J Immunol. 1990;145:2833–2838. [PubMed] [Google Scholar]
- 18.Cumberbatch M, Illingworth I, Kimber I. Immunology. 1991;74:139–145. [PMC free article] [PubMed] [Google Scholar]
- 19.Muller H K, Bucana C D, Kripke M L, Cox P A, Saijo S, Strickland F M. Cell Immunol. 1994;157:263–276. doi: 10.1006/cimm.1994.1221. [DOI] [PubMed] [Google Scholar]
- 20.Toews G B, Bergstresser P R, Streilein J W, Sullivan S. J Immunol. 1980;124:445–453. [PubMed] [Google Scholar]
- 21.Elmets C A, Bergstresser P R, Tigelaar R E, Wood J P, Streilein J W. J Exp Med. 1983;158:781–794. doi: 10.1084/jem.158.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okamoto H, Kripke M L. Proc Natl Acad Sci USA. 1987;84:3841–3845. doi: 10.1073/pnas.84.11.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saijo S, Bucana C D, Ramirez K M, Kripke M L, Strickland F M. Cell Immunol. 1995;164:189–202. doi: 10.1006/cimm.1995.1161. [DOI] [PubMed] [Google Scholar]
- 24.Simon J C, Cruz P D, Bergstresser P R, Tigelaar R E. J Immunol. 1990;145:2087–2091. [PubMed] [Google Scholar]
- 25.Simon J C, Tigelaar R E, Bergstresser P R, Edelbaum D, Cruz P D. J Immunol. 1991;146:485–491. [PubMed] [Google Scholar]
- 26.Vink A A, Sontag Y, de Gruijl F R, Roza L, Baan R A. Photodermatol Photoimmunol Photomed. 1994;10:8–12. [PubMed] [Google Scholar]
- 27.Vink A A, Strickland F M, Bucana C, Cox P A, Roza L, Yarosh D B, Kripke M L. J Exp Med. 1996;183:1491–1500. doi: 10.1084/jem.183.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ullrich S E. Photochem Photobiol. 1995;62:389–401. doi: 10.1111/j.1751-1097.1995.tb02359.x. [DOI] [PubMed] [Google Scholar]
- 29.Nishigori C, Yarosh D B, Ullrich S E, Vink A A, Bucana C D, Roza L, Kripke M L. Proc Natl Acad Sci USA. 1996;93:10354–10359. doi: 10.1073/pnas.93.19.10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yarosh D, Bucana C D, Cox P, Alas L, Kibitel J, Kripke M. J Invest Dermatol. 1994;103:461–468. doi: 10.1111/1523-1747.ep12395551. [DOI] [PubMed] [Google Scholar]
- 31.Yasui A, Takao M, Oikawa A, Kiener A, Walsh C T, Eker A P M. Nucleic Acids Res. 1988;16:4447–4463. doi: 10.1093/nar/16.10.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setlow R B, Carrier W L, Bollum F J. Proc Natl Acad Sci USA. 1965;53:1111–1118. doi: 10.1073/pnas.53.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alcalay J, Ullrich S E, Kripke M L. Photochem Photobiol. 1989;50:217–220. doi: 10.1111/j.1751-1097.1989.tb04151.x. [DOI] [PubMed] [Google Scholar]
- 34.Ceccoli J, Rosales N, Tsimis J, Yarosh D B. J Invest Dermatol. 1989;93:190–194. doi: 10.1111/1523-1747.ep12277569. [DOI] [PubMed] [Google Scholar]
- 35.Yarosh D B, Yee V. J Photochem Photobiol Biol. 1990;7:173–179. doi: 10.1016/1011-1344(90)85154-o. [DOI] [PubMed] [Google Scholar]
- 36.Roza L, Van der Wulp K J M, Mac Farlane S J, Lohman P H M, Baan R A. Photochem Photobiol. 1988;46:627–634. doi: 10.1111/j.1751-1097.1988.tb02873.x. [DOI] [PubMed] [Google Scholar]
- 37.Julius M H, Simpson E, Herzenberg L A. Eur J Immunol. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 38.Brown E, Rivas J M, Ullrich S E, Young C R, Norris S J, Kripke M L. Eur J Immunol. 1995;24:3017–3022. doi: 10.1002/eji.1830251105. [DOI] [PubMed] [Google Scholar]
- 39.Simon J C, Mosmann T R, Edelbaum D, Schopf E, Bergstresser P R, Cruz P D., Jr Photodermatol Photoimmunol Photomed. 1994;10:206–211. [PubMed] [Google Scholar]
- 40.Green M, Waugh A P W, Lowe J E, Harcourt S A, Cole J, Arlett C F. Mutat Res. 1994;315:25–32. doi: 10.1016/0921-8777(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 41.Donahue B A, Yin S, Taylor J-S, Reines D, Hanawalt P C. Proc Natl Acad Sci USA. 1994;91:8502–8506. doi: 10.1073/pnas.91.18.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fornace A J., Jr Annu Rev Genet. 1992;26:507–526. doi: 10.1146/annurev.ge.26.120192.002451. [DOI] [PubMed] [Google Scholar]