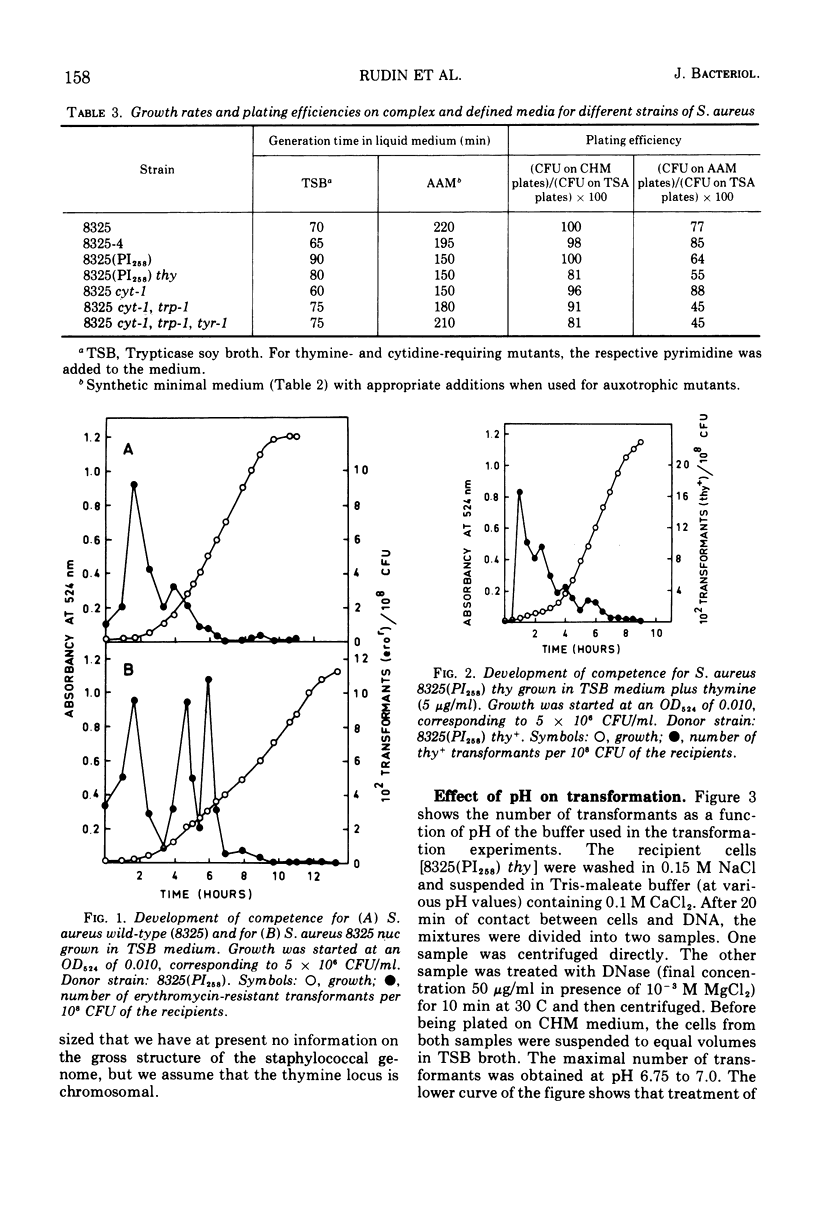
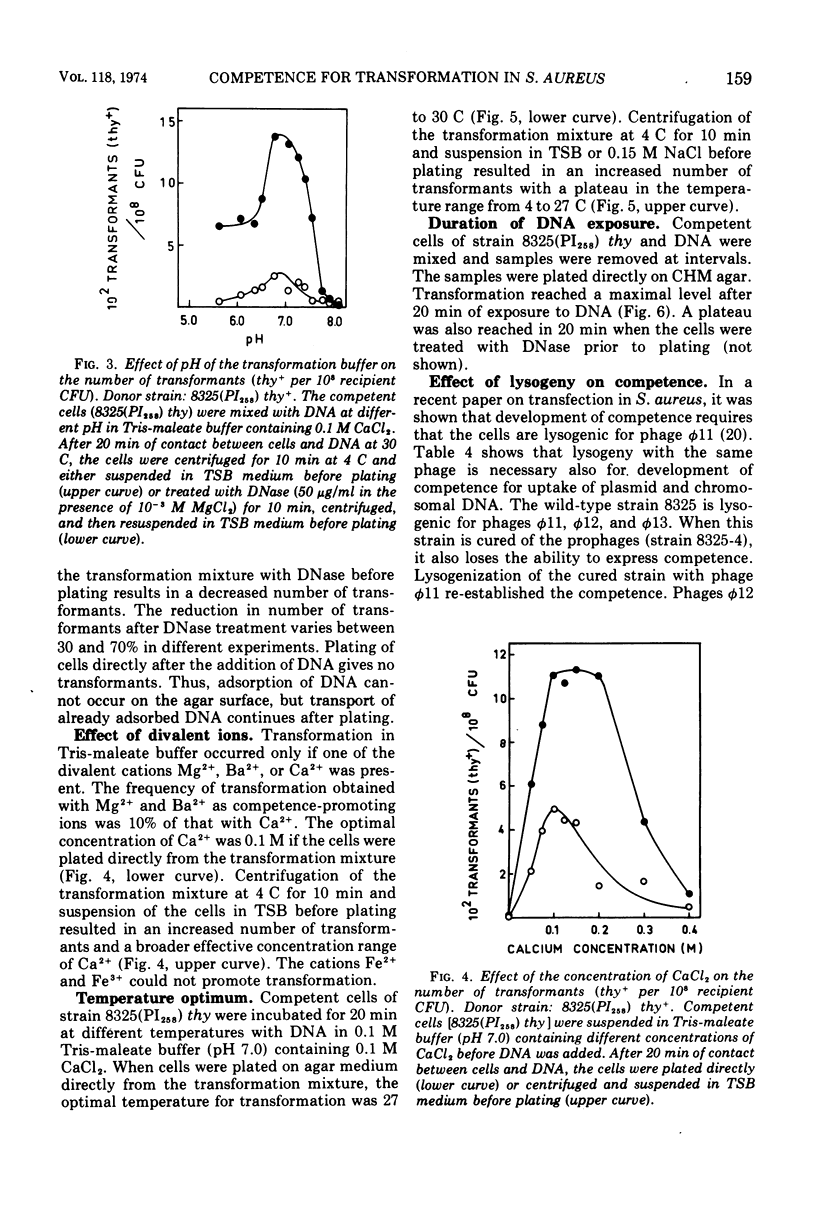
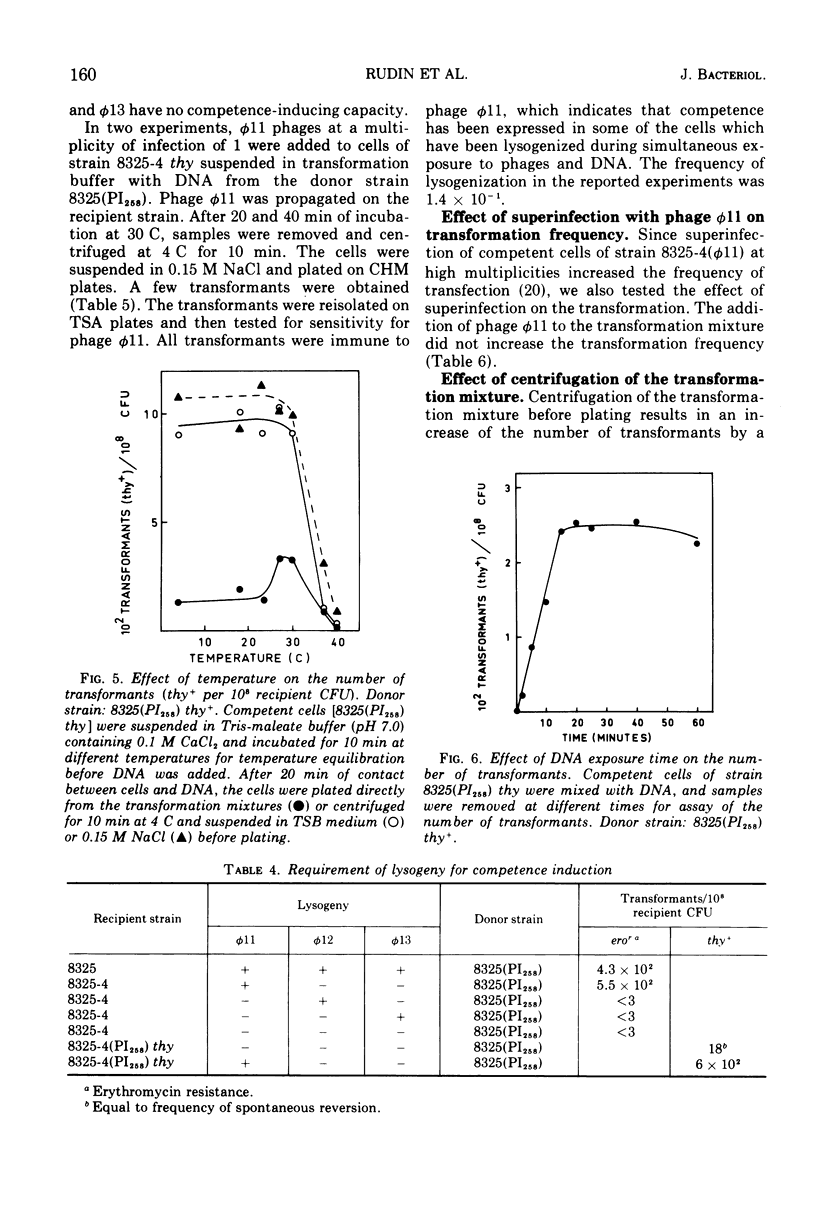
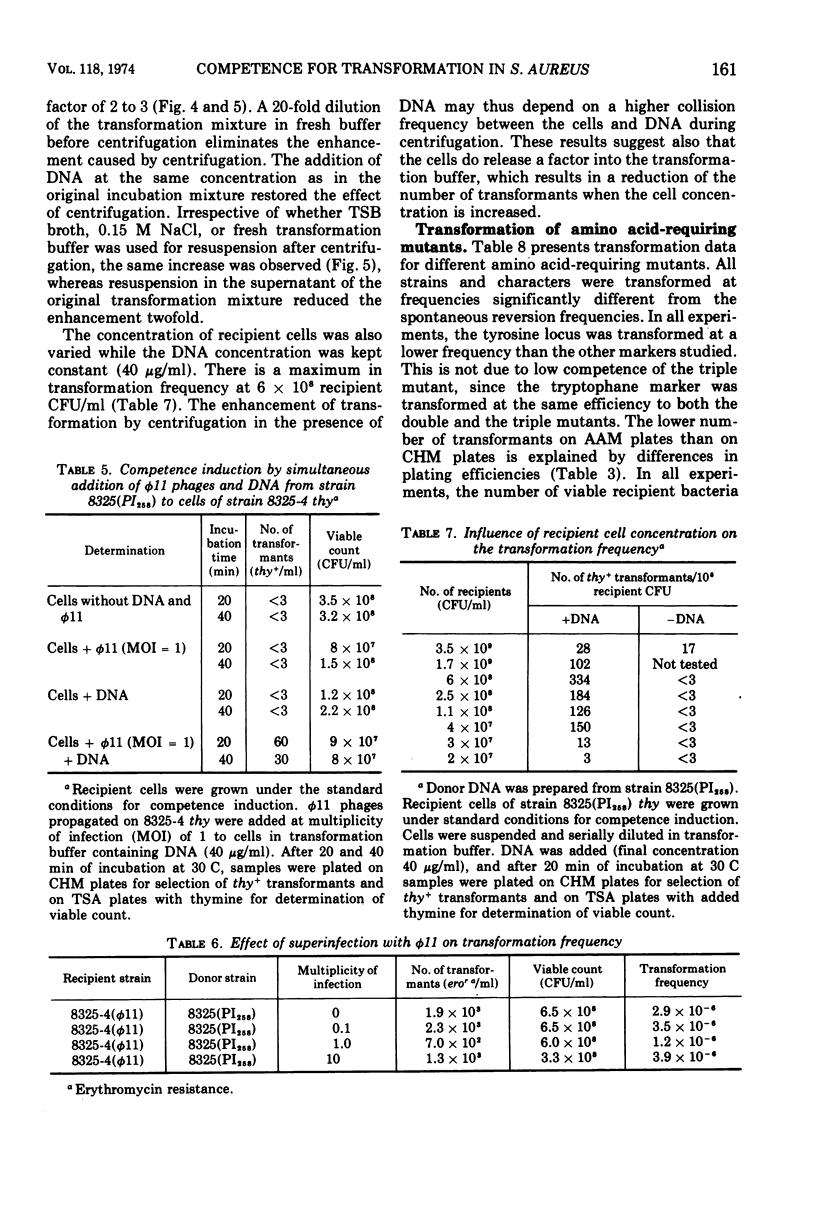
Abstract
A chemically defined medium has been developed for isolation of amino acid-requiring mutants of Staphylococcus aureus strain 8325, and for use as a selective medium in transformation assays. Variables affecting transformation of both plasmid and chromosomal markers have been studied. The optimal pH and temperature for transformation are 6.75 to 7.0 and 30 C, respectively. Ca ions are required for transformation, and only cells lysogenic for the phage φ11 can be transformed. Superinfection of competent cells with φ11 does not increase the transformation frequency. Maximal number of transformants is obtained after 20 min of contact between cells and deoxyribonucleic acid. The transformation frequencies for the plasmid marker erythromycin resistance (ero) and the chromosomal markers trp, thy, and cyt are of the same order of magnitude, whereas the frequency for the chromosomal marker tyr is approximately one order of magnitude lower.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenbern R. A. An expanded genomic map of Staphylococcus aureus. Can J Microbiol. 1971 Sep;17(9):1239–1242. doi: 10.1139/m71-197. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosloy S. D., Oishi M. The nature of the transformation process in Escherichia coli K12. Mol Gen Genet. 1973 Jul 31;124(1):1–10. doi: 10.1007/BF00267159. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R. D. CYCLICAL BEHAVIOR IN PNEUMOCOCCAL GROWTH AND TRANSFORMABILITY OCCASIONED BY ENVIRONMENTAL CHANGES. Proc Natl Acad Sci U S A. 1954 Feb;40(2):49–55. doi: 10.1073/pnas.40.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos W. E. Factors affecting transformation of Micrococcus lysodeikticus. J Bacteriol. 1969 Jun;98(3):1397–1399. doi: 10.1128/jb.98.3.1397-1399.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg M., Novick R. P. Plasmid-specific transformation in Staphylococcus aureus. J Bacteriol. 1973 Jul;115(1):139–145. doi: 10.1128/jb.115.1.139-145.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg M., Sjöström J. E., Johansson T. Transformation of chromosomal and plasmid characters in Staphylococcus aureus. J Bacteriol. 1972 Feb;109(2):844–847. doi: 10.1128/jb.109.2.844-847.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah R. A., Fung D. Y., Morse S. A. Nutritional requirements of Staphylococcus aureus S-6. Appl Microbiol. 1967 Jul;15(4):866–870. doi: 10.1128/am.15.4.866-870.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Miller R. D., Fung D. Y. Amino acid requirements for the production of enterotoxin B by Staphylococcus aureus S-6 in a chemically defined medium. Appl Microbiol. 1973 May;25(5):800–806. doi: 10.1128/am.25.5.800-806.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Oishi M., Cosloy S. D. The genetic and biochemical basis of the transformability of Escherichia coli K12. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1568–1572. doi: 10.1016/0006-291x(72)90520-7. [DOI] [PubMed] [Google Scholar]
- Omenn G. S., Friedman J. Isolation of mutants of Staphylococcus aureus lacking extracellular nuclease activity. J Bacteriol. 1970 Mar;101(3):921–924. doi: 10.1128/jb.101.3.921-924.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons L. C., Ranhand J. M., Leonard C. G., Colon A. E., Cole R. M. Inhibition of transformation in group H streptococci by lysogeny. J Bacteriol. 1973 Mar;113(3):1217–1222. doi: 10.1128/jb.113.3.1217-1222.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A. M., Rutberg L. Linked transformation of bacterial and prophage markers in Bacillus subtilis 168 lysogenic for bacteriophage phi 105. J Bacteriol. 1969 Jun;98(3):874–877. doi: 10.1128/jb.98.3.874-877.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Allison D. P., Beattie K. L. Relationship between prophage induction and transformation in Haemophilus influenzae. J Bacteriol. 1973 Jul;115(1):153–161. doi: 10.1128/jb.115.1.153-161.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotnak F. M. Marked enhancement of diplococcus pneumoniae competence for transformation by tryptic peptides of casein. Biochem Biophys Res Commun. 1971 Oct 1;45(1):63–69. doi: 10.1016/0006-291x(71)90050-7. [DOI] [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Competence for transfection in Staphylococcus aureus. J Bacteriol. 1973 Feb;113(2):576–585. doi: 10.1128/jb.113.2.576-585.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Transfection of Staphylococcus aureus with bacteriophage deoxyribonucleic acid. J Bacteriol. 1972 Jan;109(1):285–291. doi: 10.1128/jb.109.1.285-291.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J., Reilly B. E., Evans A. H. Microbial transformation and transfection. Annu Rev Microbiol. 1966;20:371–400. doi: 10.1146/annurev.mi.20.100166.002103. [DOI] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J. INCORPORATION OF DEOXYRIBONUCLEIC ACID IN THE BACILLUS SUBTILIS TRANSFORMATION SYSTEM. J Bacteriol. 1963 Sep;86:392–400. doi: 10.1128/jb.86.3.392-400.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis 168. J Bacteriol. 1973 Feb;113(2):540–548. doi: 10.1128/jb.113.2.540-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Young F. E. The influence of temperate bacteriophage phi105 on transformation and transfection in Bacillus subtilis. Biochem Biophys Res Commun. 1972 Apr 28;47(2):365–371. doi: 10.1016/0006-291x(72)90722-x. [DOI] [PubMed] [Google Scholar]


