Abstract
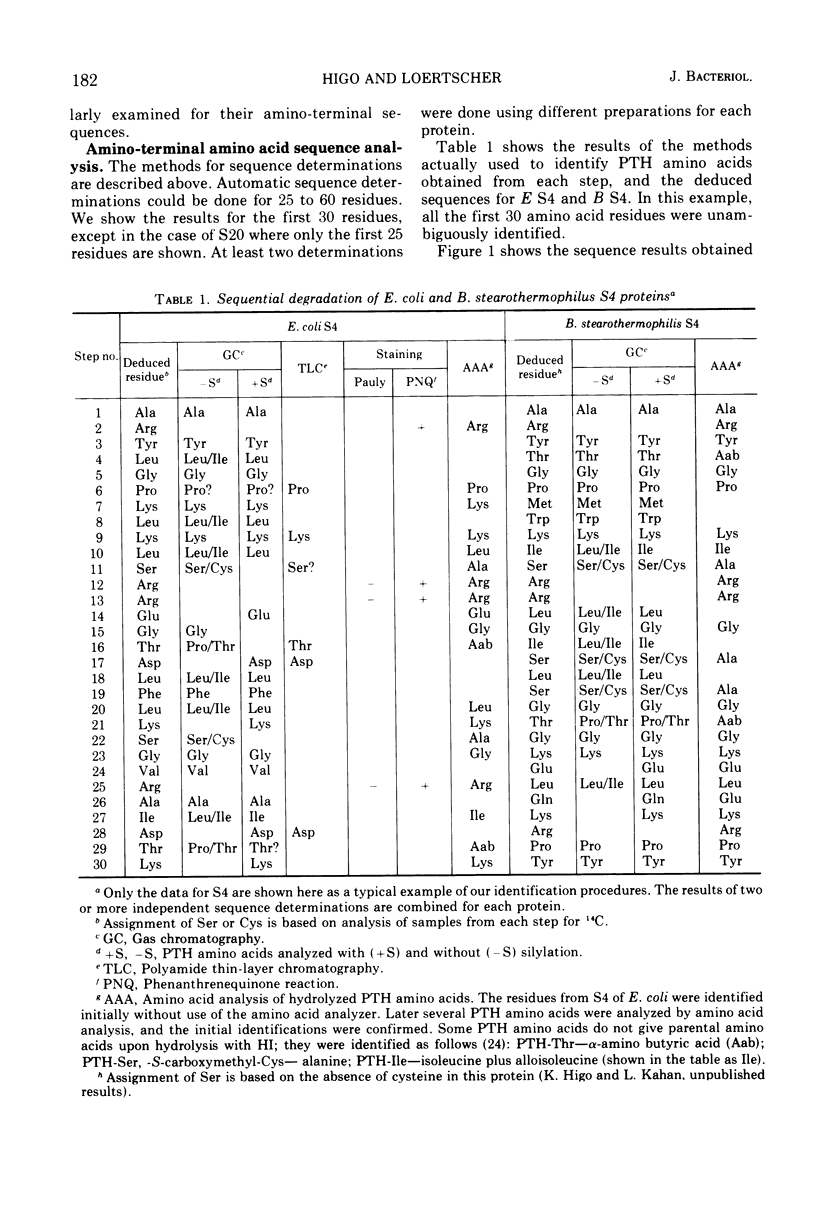
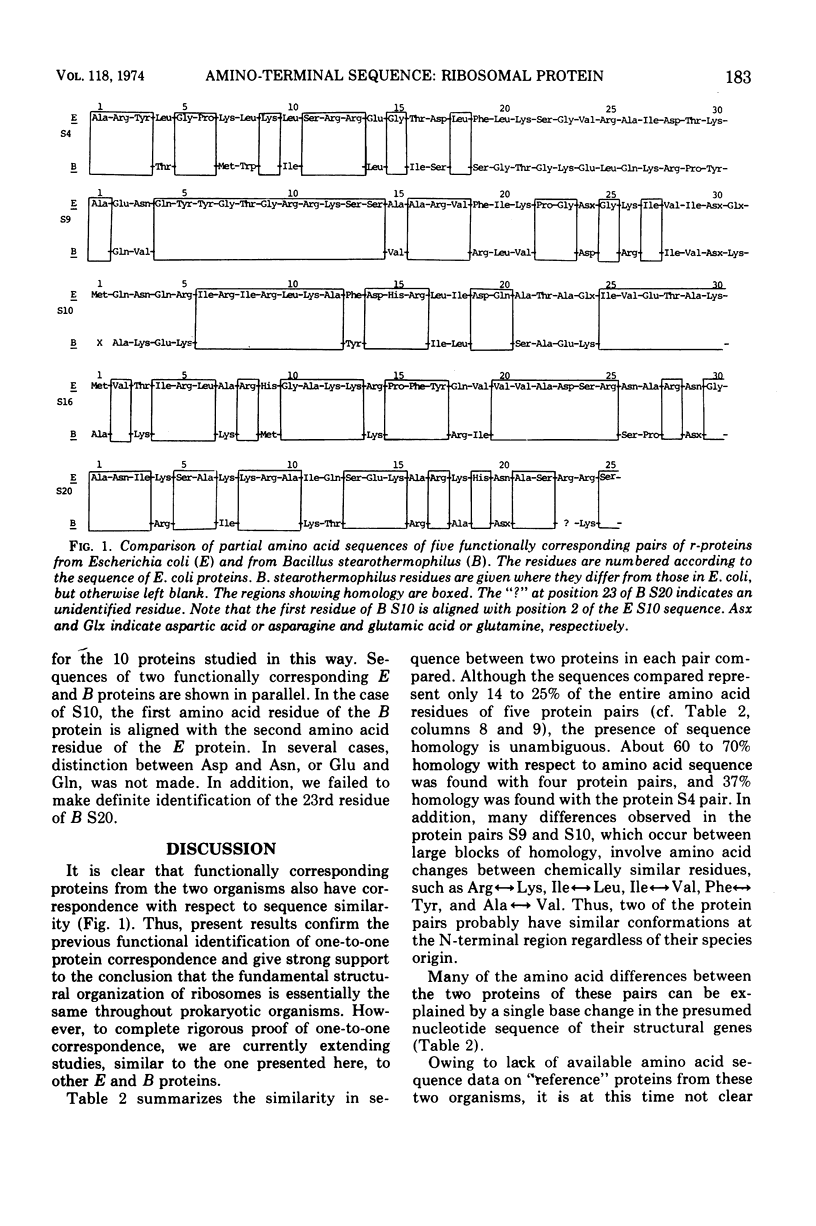
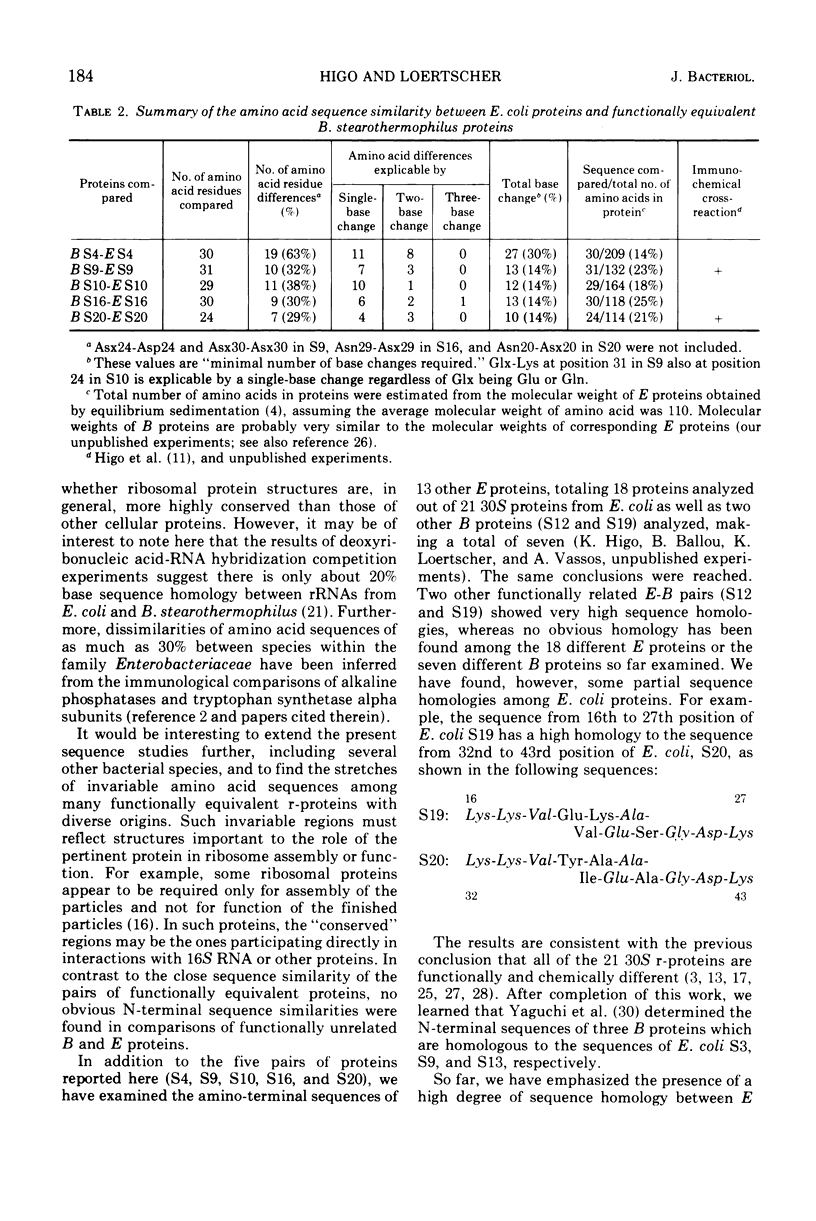
Amino-terminal sequences of five purified Escherichia coli 30S ribosomal proteins (S4, S9, S10, S16, and S20) were compared with those of their functionally corresponding Bacillus stearothermophilus ribosomal proteins identified previously by the reconstitution technique. An automatic Edman degradation method was used for sequence determinations. The sequence of the first 30 residues is presented, except that only the first 25 residues are shown for the S20 pair. Substantial (40 to 70%) sequence homologies have been observed in every case. The results show that the pairs of functionally equivalent proteins, previously identified by the reconstitution technique, are also chemically related. Thus, the present chemical studies give further support for the previous conclusion that two ribosomes with different properties, 30S subunits from E. coli and B. stearothermophilus, have the same fundamental structural organization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansley S. B., Campbell L. L., Sypherd P. S. Isolation and amino acid composition of ribosomal proteins from Bacillus stearothermophilus. J Bacteriol. 1969 May;98(2):568–572. doi: 10.1128/jb.98.2.568-572.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks G. T., Wilson A. C. Enzyme evolution in the Enterobacteriaceae. J Bacteriol. 1972 Jun;110(3):793–802. doi: 10.1128/jb.110.3.793-802.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven G. R., Voynow P., Hardy S. J., Kurland C. G. The ribosomal proteins of Escherichia coli. II. Chemical and physical characterization of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2906–2915. doi: 10.1021/bi00835a032. [DOI] [PubMed] [Google Scholar]
- Dzionara M., Kaltschmidt E., Wittmann H. G. Ribosomal proteins. 8. Molecular weights of isolated ribosomal proteins of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1909–1913. doi: 10.1073/pnas.67.4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easley C. W. Combinations of specific color reactions useful in the peptide mapping technique. Biochim Biophys Acta. 1965 Sep 13;107(2):386–388. doi: 10.1016/0304-4165(65)90147-9. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Edman P. Sequence determination. Mol Biol Biochem Biophys. 1970;8:211–255. doi: 10.1007/978-3-662-12834-3_8. [DOI] [PubMed] [Google Scholar]
- Erdmann V. A., Fahnestock S., Higo K., Nomura M. Role of 5S RNA in the functions of 50S ribosomal subunits. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2932–2936. doi: 10.1073/pnas.68.12.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. M. Protein-synthesizing machinery of thermophilic bacteria. Bacteriol Rev. 1968 Mar;32(1):27–38. doi: 10.1128/br.32.1.27-38.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held W. A., Mizushima S., Nomura M. Reconstitution of Escherichia coli 30 S ribosomal subunits from purified molecular components. J Biol Chem. 1973 Aug 25;248(16):5720–5730. [PubMed] [Google Scholar]
- Higo K., Held W., Kahan L., Nomura M. Functional correspondence between 30S ribosomal proteins of Escherichia coli and Bacillus stearothermophilus. Proc Natl Acad Sci U S A. 1973 Mar;70(3):944–948. doi: 10.1073/pnas.70.3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt E., Dzionara M., Wittmann H. G. Ribosomal proteins. XV. Amino acid compositions of isolated ribosomal proteins from 30S and 50S subunits of Escherichia coli. Mol Gen Genet. 1970;109(4):292–297. doi: 10.1007/BF00267698. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. VII. Two-dimensional polyacrylamide gel electrophoresis for fingerprinting of ribosomal proteins. Anal Biochem. 1970 Aug;36(2):401–412. doi: 10.1016/0003-2697(70)90376-3. [DOI] [PubMed] [Google Scholar]
- Kulbe K. D. Rapid separation of phenylthiohydantoin (PTH) amino acids by thin-layer chromatography on polyamide glass plates. Anal Biochem. 1971 Dec;44(2):548–558. doi: 10.1016/0003-2697(71)90244-2. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Specificity in bacterial protein synthesis: role of initiation factors and ribosomal subunits. Nature. 1970 May 23;226(5247):705–707. doi: 10.1038/226705a0. [DOI] [PubMed] [Google Scholar]
- Nomura M. Assembly of bacterial ribosomes. Science. 1973 Mar 2;179(4076):864–873. doi: 10.1126/science.179.4076.864. [DOI] [PubMed] [Google Scholar]
- Nomura M., Mizushima S., Ozaki M., Traub P., Lowry C. V. Structure and function of ribosomes and their molecular components. Cold Spring Harb Symp Quant Biol. 1969;34:49–61. doi: 10.1101/sqb.1969.034.01.009. [DOI] [PubMed] [Google Scholar]
- Nomura M., Traub P., Bechmann H. Hybrid 30S ribosomal particles reconstituted from components of different bacterial origins. Nature. 1968 Aug 24;219(5156):793–799. doi: 10.1038/219793b0. [DOI] [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- Pace B., Campbell L. L. Correlation of maximal growth temperature and ribosome heat stability. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1110–1116. doi: 10.1073/pnas.57.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace B., Campbell L. L. Homology of ribosomal ribonucleic acid diverse bacterial species with Escherichia coli and Bacillus stearothermophilus. J Bacteriol. 1971 Aug;107(2):543–547. doi: 10.1128/jb.107.2.543-547.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisano J. J., Bronzert T. J. Analysis of amino acid phenylthiohydantoins by gas chromatography. J Biol Chem. 1969 Oct 25;244(20):5597–5607. [PubMed] [Google Scholar]
- Slobin L. I., Singer S. J. The specific cleavage of immunoglobulin polypeptide chains at cysteinyl residues. J Biol Chem. 1968 Apr 25;243(8):1777–1786. [PubMed] [Google Scholar]
- Smithies O., Gibson D., Fanning E. M., Goodfliesh R. M., Gilman J. G., Ballantyne D. L. Quantitative procedures for use with the Edman-Begg sequenator. Partial sequences of two unusual immunoglobulin light chains, Rzf and Sac. Biochemistry. 1971 Dec 21;10(26):4912–4921. doi: 10.1021/bi00802a013. [DOI] [PubMed] [Google Scholar]
- Stöffler G., Wittmann H. G. Sequence differences of Escherichia coli 30S ribosomal proteins as determined by immunochemical methods. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2283–2287. doi: 10.1073/pnas.68.9.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Bickle T. A., Traut R. R. Similarity in the size and number of ribosomal proteins from different prokaryotes. J Bacteriol. 1972 Aug;111(2):474–480. doi: 10.1128/jb.111.2.474-480.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P., Hosokawa K., Craven G. R., Nomura M. Structure and function of E. coli ribosomes, IV. Isolation and characterization of functionally active ribosomal proteins. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2430–2436. doi: 10.1073/pnas.58.6.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut R. R., Moore P. B., Delius H., Noller H., Tissières A. Ribosomal proteins of Escherichia coli. I. Demonstration of different primary structures. Proc Natl Acad Sci U S A. 1967 May;57(5):1294–1301. doi: 10.1073/pnas.57.5.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi M., Roy C., Matheson A. T., Visentin L. P. The amino acid sequence of the N-terminal region of some 30S ribosomal proteins from Escherichia coli and Bacillus stearothermophilus: homologies in ribosomal proteins. Can J Biochem. 1973 Aug;51(8):1215–1217. doi: 10.1139/o73-160. [DOI] [PubMed] [Google Scholar]
- Yamada S., Itano H. Phenanthrenequinone as an analytical reagent for arginine and other monosubstituted guanidines. Biochim Biophys Acta. 1966 Dec 28;130(2):538–540. doi: 10.1016/0304-4165(66)90256-x. [DOI] [PubMed] [Google Scholar]


