Abstract
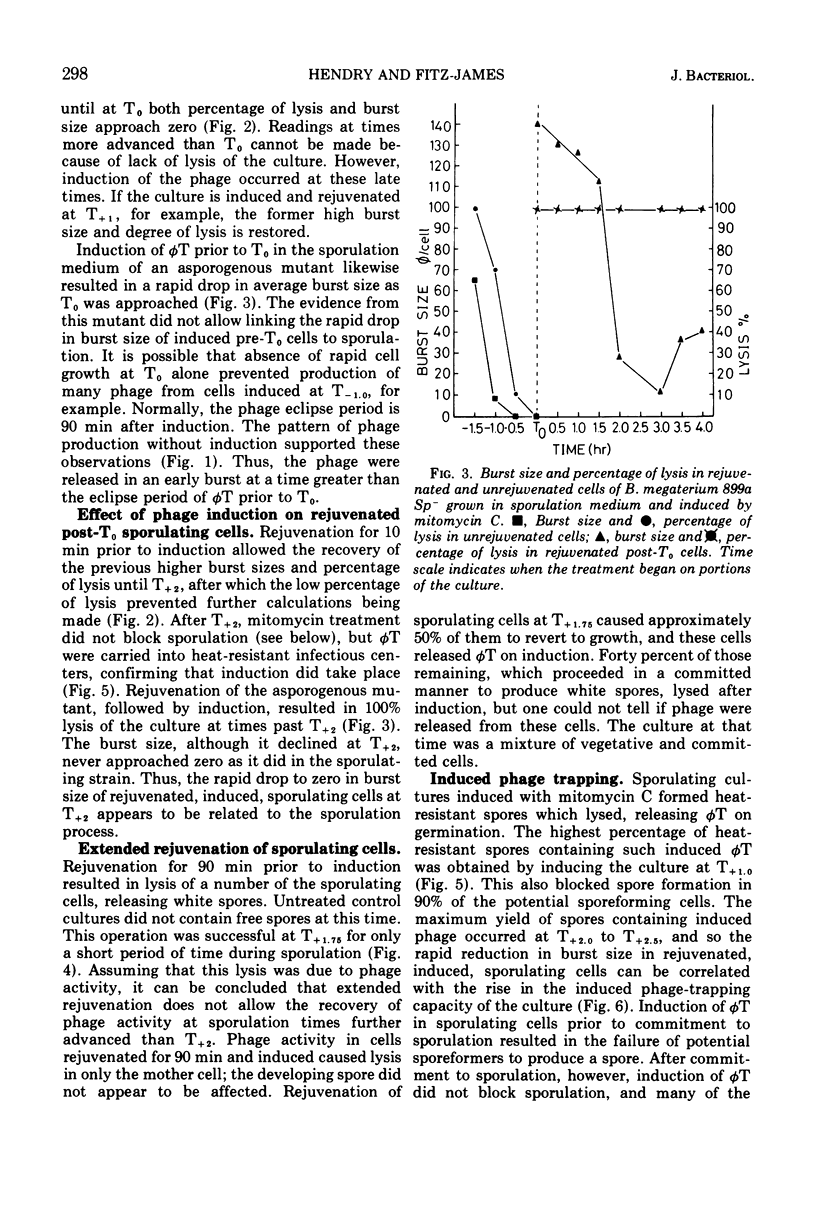
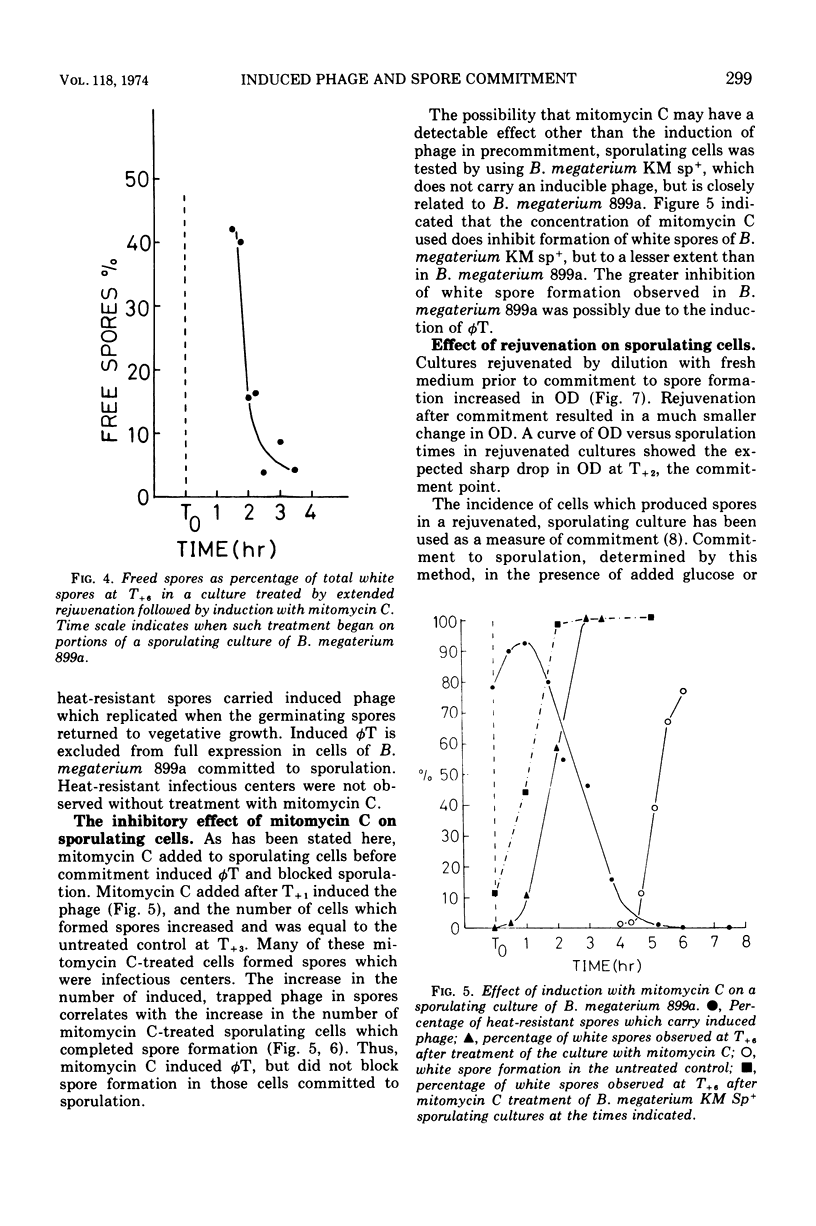
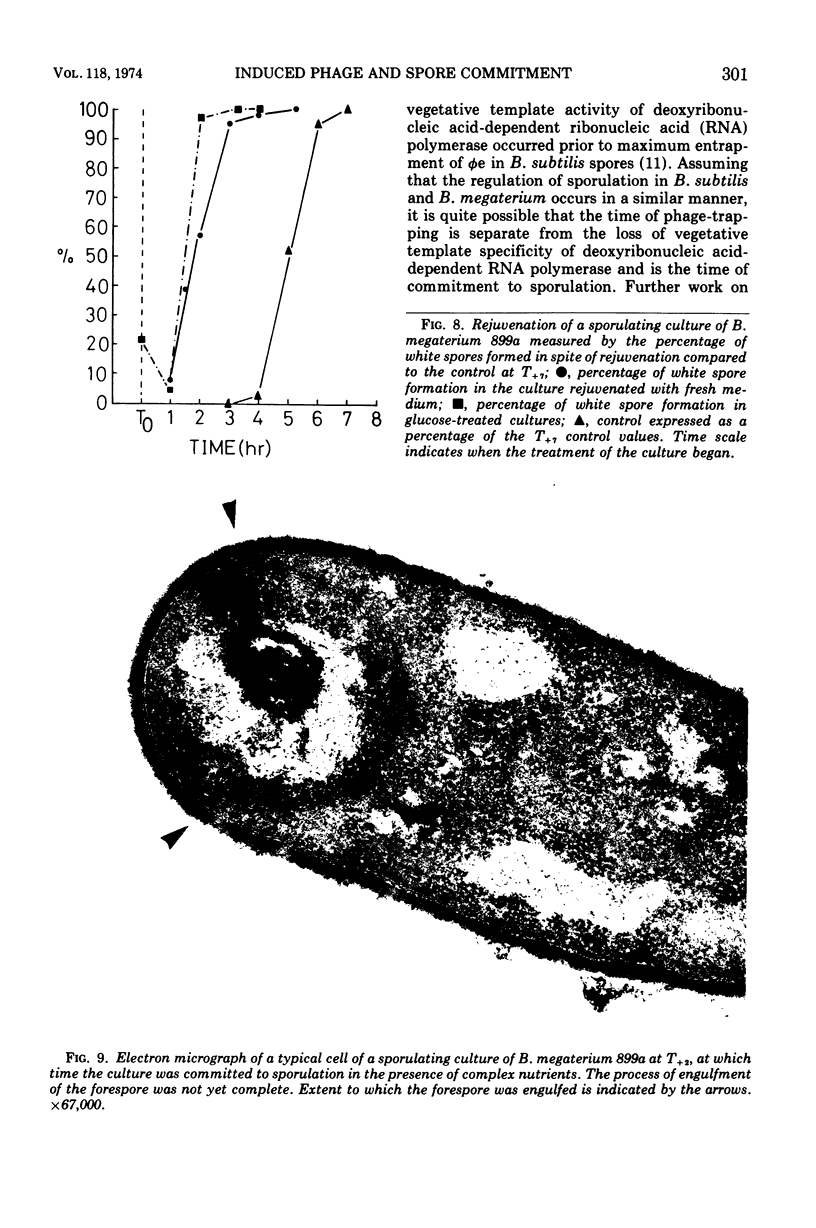
Spontaneous release of the temperate bacteriophage T (φT), carried by Bacillus megaterium 899a, occurred during early growth of the host cells. Rejuvenated cells (accomplished by a 5× dilution in fresh medium) and unrejuvenated cells were induced by mitomycin C during the course of sporulation and subsequent phage φT production measured by burst size. Induction of sequential samples of unrejuvenated cells resulted in burst sizes that fell to zero as T0 sporulation time in the main culture was approached. This drop in burst size was not considered a sporulation event, as it also occurred during analogous stages of growth in an asporogenous mutant. Rejuvenated, induced portions of the culture of sporulating cells of B. megaterium 899a gave large burst sizes until T+2, when the burst sizes fell to zero. The stage I asporogenous mutant, treated in a similar manner, gave lower, but still substantial, burst sizes; thus, the sharp decline in burst size of induced rejuvenated sporulating cells appeared to be a sporulation event. Sporulating cells induced at times shortly after T1.5 formed spores in which the induced phage were trapped until germination of the spores, which formed infectious centers. This induced phage-trapping was maximal when the sporulating cultures were induced at T2.25. Commitment to sporulation could be defined by our system as that point beyond which rejuvenated sporulating cells were unable to support the replication of the phage. This point also correlated with the increase in induced phage-trapping by spores. Two other methods gave a similar commitment time. Commitment to sporulation, in spite of added glucose or fresh complex medium, occurred at the same time. Electron micrography showed that the committed cell was still undergoing engulfment. The fate of induced phage φT was determined at different points during growth and sporulation. Induction at times prior to T0, which were longer than the eclipse period of the phage, resulted in a burst size of approximately 50. At times prior to T0, shorter than the phage eclipse period, induction led to lysis with low burst sizes, approaching zero. The pattern of spontaneous phage release during growth was similar. From T0 up to the point of commitment to sporulation, induction resulted in the blocking of spore formation without lysis. At the commitment point, induced phage were trapped and carried into spores which germinated to give infectious centers. The spontaneous derepression of phage at a time which blocked spore formation led to 7 × 104 infectious centers per ml and would not normally be noticed. Derepression at the time of phage entrapment was not observed to occur without induction with mitomycin C.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DELAPORTE B., SIMINOVITCH L. Recherches cytologiques sur un Bacillus megatherium lysogène au cours du développement du bactériophage. Ann Inst Pasteur (Paris) 1952 Jan;82(1):90–97. [PubMed] [Google Scholar]
- FITZ-JAMES P. C., YOUNG I. E. Comparison of species and yarieties of the genus Bacillus. Structure and nucleic acid content of spores. J Bacteriol. 1959 Dec;78:743–754. doi: 10.1128/jb.78.6.743-754.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Klofat W., Galliers E. Commitment to sporulation and induction of glucose-phosphoenolpyruvate-transferase. Biochim Biophys Acta. 1970 Nov 24;222(2):265–289. doi: 10.1016/0304-4165(70)90115-7. [DOI] [PubMed] [Google Scholar]
- Freese E. Sporulation of bacilli, a model of cellular differentiation. Curr Top Dev Biol. 1972;7:85–124. doi: 10.1016/s0070-2153(08)60070-8. [DOI] [PubMed] [Google Scholar]
- Fréhel C., Ryter A. Réversibilité de la sporulation chez B. subtilis. Ann Inst Pasteur (Paris) 1969 Sep;117(3):297–311. [PubMed] [Google Scholar]
- Greene R. A., Slepecky R. A. Minimal requirements for commitment to sporulation in Bacillus megaterium. J Bacteriol. 1972 Aug;111(2):557–565. doi: 10.1128/jb.111.2.557-565.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDWICK W. A., FOSTER J. W. On the nature of sporogenesis in some aerobic bacteria. J Gen Physiol. 1952 Jul;35(6):907–927. doi: 10.1085/jgp.35.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R. In vitro transcription. Annu Rev Biochem. 1972;41:409–446. doi: 10.1146/annurev.bi.41.070172.002205. [DOI] [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- MAISCH W. F., WACHSMAN J. T. THYMINELESS INDUCTION OF BACTERIOPHAGE IN BACILLUS MEGATERIUM. J Bacteriol. 1964 Nov;88:1388–1393. doi: 10.1128/jb.88.5.1388-1393.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S. M., Fitz-James P. C. Sporulation of a cortexless mutant of a variant of Bacillus cereus. J Bacteriol. 1971 Jan;105(1):339–348. doi: 10.1128/jb.105.1.339-348.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein A. L., Roscoe D. H. The course of phage phi-e infection in sporulating cells of Bacillus subtilis strain 3610. Virology. 1969 Oct;39(2):265–275. doi: 10.1016/0042-6822(69)90047-6. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI I. INCORPORATION OF BACTERIOPHAGE GENOME BY SPORES OF BACILLUS SUBTILIS. J Bacteriol. 1964 Jun;87:1499–1502. doi: 10.1128/jb.87.6.1499-1502.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG I. E. CHARACTERISTICS OF AN ABORTIVELY DISPORIC VARIANT OF BACILLUS CEREUS. J Bacteriol. 1964 Jul;88:242–254. doi: 10.1128/jb.88.1.242-254.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG I. E., FITZ-JAMES P. C. Chemical and morphological studies of bacterial spore formation. II. Spore and parasporal protein formation in Bacillus cereus var. alesti. J Biophys Biochem Cytol. 1959 Dec;6:483–498. doi: 10.1083/jcb.6.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]



