Abstract
Interleukin 16 (IL-16) has been shown to function as chemoattractant factor, as a modulator of T-cell activation, and as an inhibitor of immunodeficiency virus replication. The recent identification of inconsistencies in published IL-16 cDNA nucleotide sequences led to the proposal that IL-16 is synthesized in the form of a large precursor protein (pro-IL-16). To identify the true transcriptional start of the IL-16 mRNA rapid amplification of cDNA ends methods were applied. The complete pro-IL-16 cDNA was subsequently molecularly cloned, sequenced, and expressed in COS-7 cells. We report here that pro-IL-16 is most likely synthesized as a 67-kDa protein and is encoded from a major 2.6-kb transcript. Recombinant pro-IL-16 polypeptides are specifically cleaved in lysates of CD8(+) cells, suggesting that the naturally secreted bioactive form of IL-16 is smaller than the originally published 130 amino acids fragment. Moreover, in contrast to other interleukins such as IL-15, IL-16 mRNA expression is almost exclusively limited to lymphatic tissues underlining the potential of IL-16 as an immune regulatory molecule.
Keywords: 5′ rapid amplification of cDNA ends, full-length cDNA, precursor protein
There is ample evidence that CD8(+) cells in addition to their cytotoxic properties secrete soluble factors that inhibit HIV and simian immunodeficiency virus replication (1, 2). Furthermore, the prognosis of HIV infected individuals correlates with the ability of their CD8(+) cells isolated from HIV infected individuals to produce these antiviral factors (3). These factors may, therefore, represent important immune defense mechanisms which may be exploited in the future as part of antiviral therapeutic intervention strategies. Several secretory proteins have recently been suggested to mediate the anti-HIV activity found in supernatants of CD8(+) cell cultures, among them the chemokines RANTES, MIP-1α, and MIP-1β (4). These molecules bind to the chemokine receptor CCR5 and block virus entry of monocytotropic HIV strains which require these cell surface proteins as coreceptors (5, 6).
In addition we demonstrated in 1995 that IL-16 in the form of a 130 amino acid fragment inhibits HIV and simian immunodeficiency virus replication in primary cells (7), which was subsequently confirmed by others (8, 9). The activity of IL-16 as a lymphocyte chemoattractant is dependent on CD4 cell surface expression of the target cell (10), but experimental evidence for a direct interaction between the two proteins is still incomplete. Competition studies indicate that the IL-16 binding site on CD4 may be in close vicinity to the epitope recognized by the anti-CD4 mAb OKT4 in the V4 domain, whereas HIV gp120 binds to the V1 region of CD4 (11). The inhibitory effect of IL-16 against HIV and simian immunodeficiency virus would therefore most likely be a consequence of a signal transduction cascade initiated by crosslinking CD4 on the cell surface and not due to direct inhibition of virus entry. Recent experiments demonstrated that IL-16 is a potent inhibitor of T cell antigen receptor/CD3 mediated T cell stimulation in mixed lymphocyte reactions (12), suggesting that the antiviral properties of IL-16 may be due to alterations of the CD4+ T cell activation. Interestingly, similar effects have been shown to be evoked by certain CD4-specific mAbs (13).
In earlier investigations the good agreement between the apparent molecular mass of IL-16 in SDS/PAGE (17 kDa) and an open reading frame of 390 bp suggested that IL-16 is produced as a mature 130-amino acid protein with neither a signal peptide nor further processing (10). However, we recently found that the originally cloned IL-16 (10) is part of a much longer open reading frame ORF, indicating that the 17 kDa protein is derived from a corresponding precursor (14). Our investigation of the molecular biology of IL-16 has therefore subsequently focused on the characterization of this initially elusive IL-16 precursor.
MATERIALS AND METHODS
Cells.
Human peripheral blood mononuclear cells (PBMC) were isolated by Ficoll/Hypaque gradient centrifugation and cultivated in RPMI medium 1640 supplemented with 20% fetal calf serum, 100 units/ml IL-2 and 5 μg/ml phytohemagglutinin. T-lymphocyte subsets were prepared as described previously (2). The cell lines C8166 and COS-7 were obtained from the Medical Research Council AIDS reagent project (U.K.) and the European Collection of Animal Cell Cultures (U.K.), respectively.
RNA Preparations and Northern Blotting.
Total RNA was extracted using the RNA-Isolation kit (Stratagene). Poly(A)+ RNA was isolated from total RNA with the Oligotex-dT mRNA system (Qiagen, Chatsworth, CA). Total RNA (10 μg) or 2 μg of poly(A)+ RNA were loaded on a formaldehyde-agarose gel and after electrophoresis blotted onto a positively charged nylon membrane (Boehringer Mannheim). The IL-16 cDNA probe employed was generated using the PCR DIG-probe synthesis system (Boehringer Mannheim) and spans the IL-16 cDNA region from nucleotide 1684 to the end of the reading frame at nucleotide 2073. Hybridizations were carried out at 58°C overnight followed by several high stringency washes. For detection of the signals the DIG luminescent system (Boehringer Mannheim) was employed according to the manufacturers recommendations. The quality of RNA preparations was routinely assessed by hybridization with a β-actin probe (data not shown).
The human RNA master blot (CLONTECH) was analyzed with the same IL-16 cDNA hybridization probe under comparable conditions.
Reverse Transcription and PCR.
Identification of the 5′ end of IL-16 precursor mRNA was performed using the 5′ rapid amplification of cDNA ends (RACE) system (Life Technologies, Gaithersburg, MD). Additional RACE experiments were carried out with parts of the CapFinder system and Marathon-Ready cDNAs from human lymph nodes, leukocytes, and murine leukocytes, respectively (all from Clontech). All other cDNAs were synthesized using up to 5 μg of total PBMC RNA and oligo-dT as primer (Pharmacia).
Gel purified PCR products were ligated into the pGEM-T vector (Promega) before determination of the nucleotide sequences according to standard methods.
Oligonucleotides.
The oligonucleotides used for priming of the various cDNA synthesis reactions were cDNA1 (AAGCTCCGTGCTCGCTGG), and cDNA2 (ATGGTTCTCACTCATGAT). The primers employed in PCR amplifications were ILRC1 (GGCATCTTGAGCACTGGGCCTTG), ILRC2 (GTTGACAGCAGCCTTAGGAGCGGG), ILRC3 (ATGCAGTCAGAAATCCTAACCC), and ILRC4 (CTATAGTCCATCCGAGCCTGCCTC). The complete pro-IL-16 coding region was amplified with PBMC cDNA as template for expression cloning using the primer pair Ex2 (CTGTCTGAATTCACCTGGTATCGGCCCACAGACCAAGTC) and LFNot (GACATCGAAGCGGCCGCTGTTATTGGCTTTGGCTTC).
Peptides and Antibodies.
Antibodies specific for the pro-IL-16 derived peptides 804 (5′-KEDDDGDHSSLQSGHC-3′) and 802 (5′-RKSLQSKETTAAGDC-3′), both coupled to KLH via disulfide bonds, were raised in rabbits. The antisera and the peptides were obtained from the Custom peptide antibody production program (Eurogentec, Brussels). Recombinant IL-16 (rIL-16His) was used to raise antisera in goats (7). Affinity purified goat anti-IL-16 antibodies were used at an IgG concentration of 0.25 μg/ml in immunoblot experiments.
Immunoblots.
Cell lysates were prepared by incubation of 2.5 × 107 cells in 400 μl of solubilization buffer (20 mM Tris⋅HCl, pH 7.5/1% Nonidet P-40/150 mM NaCl/5 mM EDTA/1 mM phenylmethylsulfonyl fluoride/10 mM sodium fluoride/1 mM sodium pyrophosphate/5 μg/ml aprotinin/5 μg/ml leupeptin) for 15 min on ice. Nuclei were removed by centrifugation and the volume was finally adjusted to 500 μl with 4× SDS sample buffer. Immunoblots were carried out according to standard protocols. Antisera were used at appropriate dilutions in blocking buffer (PBS, pH 7.2/5% Marvel). Finally, bound antibody was detected using the enhanced chemoluminescence kit (Amersham).
Proteolytic Cleavage of pro-IL-16 in Cell Lysates.
Purified CD8(+) cells were lysed after cultivation for 2 days by incubation in PBS-Dulbecco/1% Nonidet P-40 for 10 min on ice. Lysates were clarified by centrifugation and finally diluted 1:5 in PBS. The equivalent of 4.5 × 106 cells was incubated with 30 μg rIL-16His in a volume of 66 μl for 16–48 hr at room temperature. Thereafter, the cleavage of rIL-16His was analyzed by immunoblotting with 1:100 diluted serum 802, which recognizes the C terminus of IL-16.
RESULTS
A 2.6-kb mRNA is the Main IL-16 Transcript and Is Predominantly Expressed in Lymphatic Tissues.
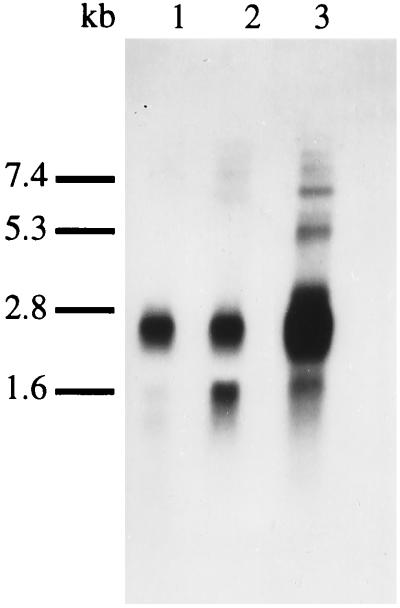
To resolve the uncertainties about the sequence and size of the IL-16 precursor we looked for IL-16 mRNA expression in PBMC and C8166 cells by Northern blot analysis using a fragment corresponding to the C terminal 390 bp as hybridization probe under stringent conditions. The major transcript in PBMCs and in the C8166 cell line is of 2.6 kb length (Fig. 1). An additional 1.6-kb transcript, readily detectable in C8166 cells, becomes visible in PBMCs only after purifying poly(A)+ RNA (Fig. 1). The larger bands in the 5–6 kb range are probably due to incompletely spliced nuclear RNAs.
Figure 1.
Northern blot analysis of pro-IL-16 mRNA expression. Lane 1, 10 μg total RNA from PBMC; lane 2, 10 μg total RNA from C8166 cells; lane 3, 2 μg purified poly(A)+ RNA from PBMC.
The human RNA tissue blot allows the direct quantitative comparison of gene expression in 50 different tissues. IL-16 mRNA was detectable at equally strong levels in spleen, thymus, and lymph node samples. Significantly lower levels of expression were seen in peripheral leukocytes, bone marrow, fetal spleen, fetal thymus, stomach, and the cerebellum. Only traces of IL-16 specific mRNA were found in Appendix, occipital lobe, salivary gland, and mammary gland tissue. Thus, 13 out of 50 tissues scored positively in this hybridization analysis. From the 13 positive tissue samples eight were of lymphatic origin including those with the highest expression levels.
Identification of the IL-16 mRNA 5′ End by Different RACE Approaches.
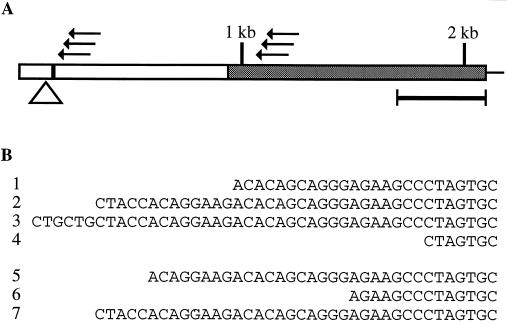
To confirm that the IL-16 precursor mRNA is indeed larger than previously described we conducted 5′ RACE experiments using an oligonucleotide located in the previously identified region of pro-IL-16 (14, 15) as primer for the cDNA synthesis (Fig. 2A). The cDNAs were tailed by use of the terminal deoxynucleotidyl transferase and finally amplified with PCR primers specific for IL-16 and the homopolymeric dC-tail (Fig. 2A). To enhance the specificity of these PCR amplifications we performed in some cases secondary nested or semi-nested PCRs prior to cloning the products. To our surprise, we found that the IL-16 cDNA sequence extends almost 1 kb beyond its previously published 5′ end (Fig. 2A and Fig. 3). However, it cannot be excluded totally that incomplete cDNAs, caused for example by secondary structures in the target RNA, had been tailed. To minimize these possibilities we used additional 5′ RACE approaches. The CapFinder system displays higher selectivity for reverse transcripts of complete mRNAs (16) and was therefore used in combination with IL-16 specific primers for cDNA synthesis to extend and confirm the sequence information obtained with the tailing approach. With both methods, the 5′ end of the pro-IL-16 cDNA was found to consist of a fairly heterogeneous set of transcriptional starting points (Fig. 2B). Similar results were obtained with commercially available cDNAs from human lymph nodes, leukocytes, and murine leukocytes, respectively (data not shown). Incompletely processed transcripts were also found containing parts of an intron just upstream of the putative initiation codon (Fig. 2A).
Figure 2.
(A) Schematic depiction of the main ORF in the IL-16 cDNA. The horizontal bar represents the originally published ORF of 390 bp (10). The shaded region represents the subsequently reported IL-16 ORF (14, 15). The locations of oligonucleotides used in RACE experiments are shown as arrows. The triangle identifies a splice junction at nucleotide position 129 where frequently short intervening sequences of variable length (80–200 bp) have been found. The putative initiation codon is shown as a black vertical bar. (B) Heterogenous starting points for the initiation of transcription of the IL-16 gene. Only the very beginning of the IL-16 cDNA 5′ end as found in different clones is shown. Clones 1–4 were obtained using parts of the CapFinder system. Clones 5–7 were generated by the tailing strategy. All sequence numbering in this report relates to the first base of clone 3 as being nucleotide 1.
Figure 3.
Nucleotide and deduced amino acid sequence of the IL-16 precursor cDNA. The two possible initiation codons are underlined. The ATG that was originally believed to mark the beginning of the 390 bp IL-16 coding region is marked by a black bar. The previously published 5′ end of the IL-16 cDNA is shown by an arrow. The location of the splice junction where in some cDNAs short intervening sequences have been found is identified by a triangle. The positions of the putative proteolytic cleavage site at aspartate residue 510 (nucleotide position 1,708), and the codon GCA (alanine) at nucleotide position 1,759, which is absent in some IL-16 splice variants, are underlined. The 3′-UTR is not shown.
Sequence of the IL-16 Precursor.
The complete nucleotide and deduced amino acid sequence of the pro-IL-16 cDNA is shown in Fig. 3. The first ATG is found at position 181 and if used as a start codon would result in a relatively hydrophylic protein of 631 amino acids with a calculated molecular mass of 67 kDa. Use of the second ATG at position 262, which is in frame and in very good context for the initiation of translation (17), would give rise to a 63 kDa protein. The putative 5′ leader sequence is G+C rich (61%) and may contribute to the formation of secondary structures.
The absence of a signal peptide for transport into the endoplasmatic reticulum leaves the mechanism of secretion for pro-IL-16 or smaller proteolytic cleavage products unexplained. As reported before, the most significant homologies in database searches were seen to the presynaptic density protein 95 and the tight junction protein ZO-1 (GenBank accession nos. P31016P31016 and Q07157Q07157, respectively). Both carry GLGF motifs and the resemblance is solely due to the three GLGF domains in the C-terminal half of the pro-IL-16 sequence (14, 15).
Database searches for the 3′ untranslated region (UTR) of the pro-IL-16 cDNA, which has a proposed length of 979 bp, were also carried out (10). The three expressed sequence tags (GenBank accession nos. N38840N38840, H57532H57532, and N22522N22522) that cover the 3′ end were found to begin in a region between nucleotides 2362 and 2376 due to utilization of the polyadenylylation signal at nucleotide position 2344. Thus, for the majority of transcripts the 3′ UTR of approximately 303 nucleotides would be significantly shorter than previously described (10).
Immunoblot Detection of pro-IL-16 in Cell Lysates.
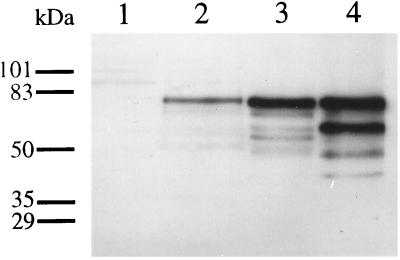
The IL-16 precursor protein was detectable in mitogen stimulated PBMCs as a protein band with an apparent molecular mass of ≈80 kDa (Fig. 4). Interestingly, in freshly isolated (data not shown) as well as in serotonin stimulated cells an almost equally strong second 60-kDa band was seen (Fig. 4). Only overexposure of films allowed detection of the same 60-kDa protein in the samples after two days of cultivation in the presence of mitogen and IL-2 (data not shown).
Figure 4.
Immunoblot detection of pro-IL-16 in cell lysates. Lysates from 2.5 × 106 cells were loaded into each lane. Lane 1, normal COS-7 cells; lane 2, pro-IL-16 cDNA transfected COS-7 cells; lane 3, phytohemagglutinin/IL-2 stimulated PBMCs; lane 4, PBMCs after 24 hr of stimulation with 10 μM serotonin.
To verify that the pro-IL-16 cDNA (Fig. 3) can be expressed, the pro-IL-16 coding region under transcriptional control of the CMV promoter (pcDNA3, Invitrogen) was transfected into COS-7 cells. Immunoblots with lysates from transfected cells and PBMCs revealed that transfected cells, but not untransfected controls, express a 80-kDa protein that migrates in a manner identical to the pro-IL-16 found in PBMCs (Fig. 4). Similar results were seen with pro-IL-16 specific serum 804 whereas use of preimmunization sera gave no signals (data not shown).
Proteolytic Cleavage of pro-IL-16 in Cell Lysates.
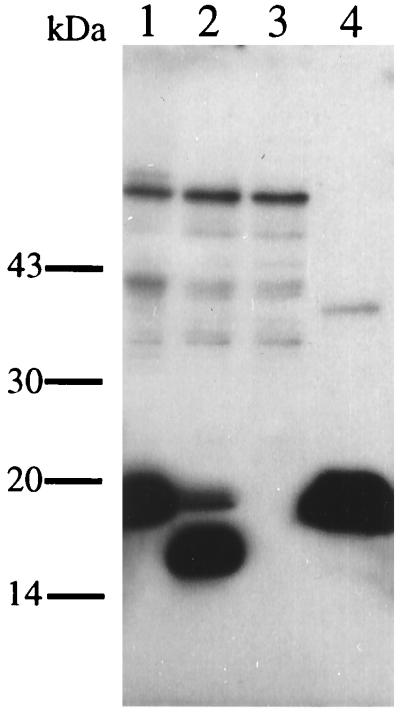
Theoretically, the IL-16 precursor protein should be a substrate for proteases present in or on CD8(+) cells, the action of which would release the biologically active C-terminal portion. A recombinant pro-IL-16 fragment of 39 kDa (14) is specifically proteolytically processed upon incubation in CD8(+) cell lysates and not in lysates from CD4(+) cells (data not shown). As there is no clear evidence to support the assumption that the 130-amino acid form of IL-16 is identical to the IL-16 naturally found in cell culture supernatants or body fluids, we asked whether the recombinant 130-amino acid fragment would still act as a substrate for this proteolytic activity (Fig. 5). Indeed, incubation of rIL-16His, which migrates as a 19 kDa protein in SDS gels, with CD8(+) cell lysate yields the same 17 kDa C-terminal fragment as seen previously in the 39-kDa precursor variant cleavage assays. No protease activity is detectable during the incubation time without addition of cell lysate (Fig. 5). Therefore it is likely that naturally processed IL-16 is smaller than the originally suggested 130 amino acids. Preliminary laser desorption mass spectroscopy data obtained with purified cleavage products indicate that proteolytic processing occurs at the aspartate residue 510 (Fig. 3).
Figure 5.
Proteolytic cleavage of rIL-16His in lysates of CD8(+) cells. Lane 1, rIL-16His in lysate after 0 min incubation; lane 2, rIL-16His6 in lysate after a 24 hr incubation; lane 3, lysate without added protein; lane 4, rIL-16His6 after a 24 hr incubation in identical buffer conditions but no added lysate. Note the aberrant migration behavior of rIL-16His, which has a calculated molecular mass of 15 kDa, and of the cleaved product.
DISCUSSION
In cytokine research, numerous precedents underline that exact knowledge of precursor-product relationships may be essential to identify (often pleiotropic) functions. For example, the IL-1alpha precursor is already fully functional (18), whereas for IL-1beta only the mature C-terminal processing product displays biological activity (19). Once it was realized that the originally described 130-amino acid IL-16 protein (10) represents only an artificially generated part of a much larger precursor it became mandatory to identify the complete pro-IL-16 cDNA sequence to establish a rational basis for studies of the pro-IL-16 protein and its naturally processed product(s).
The main pro-IL-16 transcript is a 2.6 kb mRNA (Fig. 1). Surprisingly, the IL-16 cDNA sequence was found to extend ≈1 kb beyond its previously published 5′ end (Figs. 2 and 3). Sequence analysis of the heterogeneous pro-IL-16 cDNA 5′ ends indicates the presence of a fairly long G-C rich 5′-UTR which may affect the translation in vivo by formation of secondary structures. With a likely 3′ UTR of ≈303 nucleotides the pro-IL-cDNA would be 2376 bp in length, which is in good agreement with the Northern blot results.
The control of pro-IL-16 expression is likely to be unusually complex as (i) most tissues are negative for pro-IL-16 transcripts (ii) CD4(+) cells carry IL-16 RNA but protein synthesis is only initiated upon mitogen stimulation and (iii) CD8(+) cells produce the protein constitutively (20). Incompletely spliced cDNAs led to the identification of an intron in the putative 5′ UTR that may contribute to the translational control of pro-IL-16 expression by the introduction of short upstream ORFs (17).
Upon transfection of molecularly cloned pro-IL-16 cDNA into COS-7 cells a 80 kDa protein was detectable that migrates identically to the major proIL-16 form shown in PBMC lysates (Fig. 4), suggesting that indeed the complete pro-IL-16 cDNA was expressed. The discrepancy between a calculated molecular mass of 63 or 67 kDa for pro-IL-16 (depending on the utilized initiation codon), and an apparent molecular mass of 80 kDa is not surprising since an aberrant migration behavior in SDS/PAGE gels is a common property of IL-16 derived polypeptides (Fig. 4). Furthermore, it remains to be seen whether pro-IL-16 is posttranslationally modified.
It is also unknown at present whether the two forms of pro-IL-16 detected as 80 kDa and 60 kDa proteins in immunoblots using lysates from serotonin stimulated cells (Fig. 4) and freshly isolated PBMC result from proteolytic processing, differential splicing or alternative promoter usage. Both forms could serve as functional precursors as they are reactive with serum specific for the C terminus of IL-16. The biological functions of pro-IL-16 remain to be addressed in future investigations.
Supposing that pro-IL-16 is a 67 kDa protein of 631 amino acids and its C-terminal portion purified from cell culture supernatants migrates as 17 kDa protein (10) there are now numerous open questions to answer. That the pro-IL-16 protein is subject to proteolytic processing was shown by incubation of recombinant IL-16 polypeptides with cell lysates from CD8(+) cells (Fig. 5). One likely cleavage site is at the aspartate residue 510 and would lead to a fragment of 121 amino acids with a calculated isoelectric point of 4.9. Interestingly, the protease family of IL-1 converting enzymes and granzyme B are known to require a C-terminal aspartate residue as part of their recognition sites (21, 22).
Availability of the most likely complete pro-IL-16 cDNA, which is efficiently expressed in transfected COS-7 cells, will open further routes toward a better understanding of the biology of pro-IL-16 and its secreted C-terminal portion. In addition to studying the obviously pleiotropic biological functions (11) of properly folded and processed IL-16, this system should help both the identification of cellular proteases involved in IL-16 processing and investigations into the mechanism of IL-16 secretion.
Acknowledgments
We thank Tanja Kearns, Sylvia Raupp, Andrea Wangorsch, and Gudrun Winskowsky for excellent technical assistence, Dr. Manfred Wozny for his help with the mass spectroscopy measurements, and Dr. Ottmar Janssen for helpful discussions. Parts of this work were supported by Grant 1506/TG04 of the Federal Ministry of Health and by Grant 01 KI 9407/9 of the Federal Ministry of Research and Technology.
ABBREVIATIONS
- IL
interleukin
- rIL
recombinant IL
- PBMC
peripheral blood mononuclear cells
- RACE
rapid amplification of cDNA ends
- UTR
untranslated region
Footnotes
References
- 1.Walker C M, Moody D J, Stites D P, Levy J A. Science. 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 2.Ennen J, Findeklee H, Dittmar M T, Norley S, Ernst M, Kurth R. Proc Natl Acad Sci USA. 1994;91:7207–7211. doi: 10.1073/pnas.91.15.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy J A. AIDS. 1993;7:1401–1410. doi: 10.1097/00002030-199311000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 5.Deng H K, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Marzio P D, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall C J, Littman D R, Landau N R. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 6.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 7.Baier M, Werner A, Bannert N, Metzner K, Kurth R. Nature (London) 1995;378:563. doi: 10.1038/378563a0. [DOI] [PubMed] [Google Scholar]
- 8.Levy J A, Mackewicz C E, Barker E. Immunol Today. 1996;17:217–224. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- 9.Hillyer C D, Villinger F, Brice G T, Adams J W, Lackey D A, Lee M E, Mayne A, Brar S S, Ansari A A. Annu Symp Nonhuman Primate Models AIDS. 1996;14:77. , 169 (abstr.). [Google Scholar]
- 10.Cruikshank W W, Center D M, Nisar N, Wu M, Natke B, Theodore A C, Kornfeld H. Proc Natl Acad Sci USA. 1994;91:5109–5119. doi: 10.1073/pnas.91.11.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Center D M, Kornfeld H, Cruikshank W W. Immunol Today. 1996;17:476–481. doi: 10.1016/0167-5699(96)10052-i. [DOI] [PubMed] [Google Scholar]
- 12.Theodore A C, Center D M, Nicoll J, Fine G, Kornfeld H, Cruikshank W W. J Immunol. 1996;59:1958–1964. [PubMed] [Google Scholar]
- 13.Benkirane M, Schmid-Antomarchi H, Littman D R, Hirn M, Rossi B, Devaux C. J Virol. 1995;69:6904–6910. doi: 10.1128/jvi.69.11.6904-6910.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bannert N, Baier M, Werner A, Kurth R. Nature (London) 1996;381:30. doi: 10.1038/381030a0. [DOI] [PubMed] [Google Scholar]
- 15.Bazan J F, Schall T J. Nature (London) 1996;381:29–30. doi: 10.1038/381029a0. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Y, Chenchik A, Siebert P D. Clontechniques. 1996;11:30–31. [Google Scholar]
- 17.Kozak M. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinarello C A. In: The Cytokine Handbook. Thomson A, editor. London: Academic; 1994. pp. 31–56. [Google Scholar]
- 19.Black R A, Kronheim S R, Cantrell M, Deely M C, March C J, Pricket K S. J Biol Chem. 1988;263:9437–9442. [PubMed] [Google Scholar]
- 20.Laberge S, Cruikshank W W, Kornfeld H, Center D M. J Immunol. 1995;55:2902–2910. [PubMed] [Google Scholar]
- 21.Henkart P A. Immunity. 1996;4:195–201. doi: 10.1016/s1074-7613(00)80428-8. [DOI] [PubMed] [Google Scholar]
- 22.Darmon A J, Nicholson D W, Bleackley R C. Nature (London) 1995;377:446–448. doi: 10.1038/377446a0. [DOI] [PubMed] [Google Scholar]