Abstract
Disulfiram has shown promise in several clinical trials for cocaine addiction, but its potential utility in the treatment of amphetamine addiction has not been examined. The goal of this study was to determine the effects of disulfiram on acute physiological and subjective responses to dextroamphetamine in healthy volunteers. Five male and 5 female subjects participated in an outpatient double-blind, placebo-controlled, crossover study. Subjects were randomly assigned to a sequence of disulfiram (250 mg/day) or placebo treatments each lasting for 4 days. Day four of each treatment period was the experimental session, in which subjects orally ingested a single dose of dextroamphetamine (20 mg/70 kg). Outcome measures included heart rate, blood pressure, plasma cortisol and prolactin, subjective and performance on the Sustained Attention to Response Test (SART). Disulfiram did not affect dextroamphetamine-induced increases in heart rate, blood pressure, cortisol, or prolactin. Disulfiram did enhance some of the subjective effects of dextroamphetamine including ratings of “high,” “anxious,” “bad drug effects,” “want more drug” and “drug liking” and was also associated with decreased performance in the SART test. How these enhanced subjective amphetamine responses affect cocaine use behavior remains to be determined in future clinical trials.
Disulfiram enhances subjective effects of dextroamphetamine in humans. Amphetamine abuse, especially methamphetamine, is an important public health problem in the US, with an estimated 1.4 million have reported methamphetamine use during the past year (SAMHSA, 2004). There are no approved pharmacotherapies for the treatment of amphetamine (Hill and Sofuoglu, 2007) or cocaine addiction, the other commonly used stimulant (Sofuoglu and Kosten, 2006). Given the similarities between the pharmacological effects of cocaine and amphetamines (Fischman et al., 1976), it is plausible that potential pharmacotherapies may be effective for both cocaine and amphetamine addiction. One of the promising medications for cocaine addiction is disulfiram (Antabuse), which has been shown to reduce cocaine abuse and relapse in outpatient clinical trials (Carroll et al., 1993; Carroll et al., 2004; Carroll et al., 1998; George et al., 2000; Petrakis et al., 2000). Disulfiram is approved by the Food and Drug Administration for the treatment of alcohol dependence, where its efficacy is due to the unpleasant symptoms experienced when acetaldehyde levels increase following ethanol ingestion because of disulfiram-induced aldehyde dehydrogenase inhibition (Hughes and Cook, 1997). Disulfiram also inhibits dopamine β-hydroxylase (DBH), the enzyme that converts dopamine to norepinephrine, and thereby causes synaptic dopamine levels to increase relative to norepinephrine (Karamanakos et al., 2001; Vaccari et al., 1996). This DBH inhibition may explain disulfiram’s therapeutic effects in cocaine addiction (Cubells and Zabetian, 2004).
Despite promising findings in the treatments of cocaine dependence, disulfiram has not been tested as a treatment for amphetamine addiction. The goal of this pilot study was to evaluate the potential utility of disulfiram in treating amphetamine addiction by examining its interactions with dextroamphetamine in humans, which has not been examined previously. We examined disulfiram’s effects on the physiological, endocrine, subjective, and cognitive effects of dextroamphetamine using a double-blind, placebo-controlled, crossover design.
Method
Participants
Five male and 5 female healthy controls were recruited from the New Haven area (6 African-Americans, 2 Caucasian, 1 Hispanic and 1 Asian) by newspaper advertisements and flyers. The average age (SD) of the subjects was 33.3 years (4.4). All subjects had normal physical, laboratory, and psychiatric examinations, and none were dependent on alcohol or other drugs except nicotine. In order to minimize confounding effects from nicotine or caffeine withdrawal, those who smoked more than five cigarettes or drank more than three cups of caffeinated beverages per day were excluded, following a procedure used in previous amphetamine administration studies (Lott et al., 2005). All subjects provided informed consent prior to study entry and were paid for participation. Experimental sessions were conducted in the Biostudies Unit located at the VA Connecticut Healthcare System, West Haven campus. This study was approved by the VA Connecticut Healthcare System Human Subjects Subcommittee.
Design and Procedures
In this double-blind, placebo-controlled, crossover study, subjects had two treatment periods, each lasting for 4 days. In the first treatment period, subjects were assigned randomly to receive either disulfiram (250 mg/day) or placebo for three days, during which the subjects visited the outpatient clinic at 8 AM to receive study medication and monitor medication effects. The treatment sequence was counterbalanced such that equal number of subjects received disulfiram or placebo first. On the fourth day, a laboratory session started at 8 AM and lasted for approximately six hours, during which subjects were given dextroamphetamine and another dose of either disulfiram or placebo at the same dose that they had received previously. At the start of the session, an indwelling intravenous catheter was placed in the antecubital vein both for blood drawing and as a safety precaution. Baseline measures were obtained, and then subjects were given that day’s dose of the study medication (disulfiram or placebo) and dextroamphetamine followed by a light meal. One hour after medication administration, subjects were stressed psychologically and then thirty minutes later stressed physically, which will be described elsewhere. Two hours after medication administration, repeat subjective and physiological measures were obtained, and then a 20 mg/70 kg dose of dextroamphetamine was administered. Outcome measures were collected over the remaining four hours of the experimental session. This first treatment period was followed by a four- to fifteen-day “washout” period in order to minimize residual effects from study medications.
Following this “washout” period, subjects entered a second treatment period that also lasted for four days. The procedure for this period was identical to that of the first treatment period, except that those subjects receiving disulfiram in the first period now received placebo, and vice versa.
Drugs
Dextroamphetamine (the dextrorotatory isomer of amphetamine) was obtained from Barr Laboratories (Pomona, New York). Dextroamphetamine is used for the treatment of attention-deficit hyperactivity disorder (ADHD) and narcolepsy at a dose of 5 to 60 mg/day (PDR, 2007). In this study, we used a single 20 mg/70 kg oral dose, which has been shown to produce typical amphetamine effects in healthy volunteers (Brauer and de Wit, 1996; Fillmore et al., 2003; Sofuoglu et al., 2008; Tidey et al., 2000). Plasma levels of dextroamphetamine peak within one to three hours following oral administration, and the elimination half-life is 10 to 13 hours (PDR, 2008).
Disulfiram (Odyssey Pharmaceuticals, Inc., East Hanover, New Jersey) was administered in a single dose at 250 mg/day for four days, a dose which has been previously used in clinical trials for cocaine addiction. Plasma levels of disulfiram peak within one to two hours following oral administration, and the elimination half-lives of disulfiram and its active metabolites range from four to twelve hours (Faiman et al., 1984).
To minimize the risk of disulfiram interacting adversely with alcohol (the “Antabuse reaction”), subjects were thoroughly informed of this risk and instructed to abstain from alcohol and alcohol-containing substances for at least 12 hours prior to the first drug administration and for two weeks after they completed the study. To minimize adherence concerns with the study medications, disulfiram was administered daily in the clinic by the study nurse and subjects closely monitored during ingestion. Subjects were tested daily for drug and alcohol use, with urine toxicology screening and breathalyzer, respectively.
Measures
The outcome measures included endocrine, physiological, subjective, and cognitive performance measures. The endocrine measures of plasma cortisol and prolactin were obtained at baseline and then at one, two, and three hours after dextroamphetamine administration. The measures of cortisol and prolactin were chosen as their release is controlled by the central noradrenergic and dopaminergic pathways, respectively, (Charmandari et al., 2005; Freeman et al., 2000) and both hormones have been shown to be sensitive to amphetamine administration (Grady et al., 1996; Seiden et al., 1993).
The physiological measures consisted of systolic and diastolic blood pressure and heart rate, which were measured at baseline then at 30, 60, 90, 120, 150, and 180 minutes after dextroamphetamine administration.
The subjective measures were given at baseline, and then at 30, 60, 90, 150, and 180 minutes after the medication treatment and were comprised of the Drug Effects Questionnaire (DEQ), the Addiction Research Center Inventory-Short Form (ARCI), and the Profile of Mood States (POMS). The DEQ assessed the acute subjective effects of dextroamphetamine, and asked subjects to rate “stimulated”, “high”, “anxious”, “sedated”, “down”, “feeling the drug strength”, “feel good drug effects”, “feel bad drug effects”, “want more drug”, and “like the drug” on a 100 mm scale, from 0 (“not at all”) to 100 (“extremely”). The ARCI consists of 49 true-or-false questions with five subscales: drug-induced euphoria (Morphine-Benzedrine Group; MBG), stimulant-like effects (Amphetamine; A), intellectual efficiency and energy (Benzedrine Group; BG), dysphoria (Lysergic Acid; LSD), and sedation (Pentobarbital-Chlorpromazine; PCAG) (Martin et al., 1971). The POMS is a 72–item rating scale used to measure the effects of medication treatments on mood using six subscales: (1) composed-anxious; (2) agreeable-hostile; (3) elated-depressed; (4) confident-unsure; (5) energetic-tired; and (6) clear headed-confused (McNair et al., 1971). For all subscales, higher scores indicate more positive mood states.
Cognitive performance was assessed with the Sustained Attention to Response Test (SART), which was administered two hours after dextroamphetamine administration. The SART assesses the ability to withhold automatic responses to an infrequently occurring target. Errors have been interpreted as indicating impaired sustained attention. The SART discriminates ADHD cases from controls (Shallice et al., 2002), and depressed from non-depressed men (Farrin et al., 2003), correlates with other tests of sustained attention (Robertson et al., 1997), time-of-day (Manly et al., 2000) and subjective sleepiness (Manly et al., 2002), and is sensitive to acute pharmacological challenges (Sofuoglu et al., 2008). 225 single digits (25 × 9 digits) are presented on a computer monitor for 250 ms each, immediately followed by a mask for 900 ms. Subjects must press a spacebar in response to every digit except the “3,” and to give equal importance to both speed and accuracy [see (Sofuoglu et al., 2008) for details].
Statistical Analysis
To assess treatment effects, we used a mixed-effect repeated-measures crossover analysis using SAS Proc Mixed (version 9.13). The structure of the analysis included a fixed main effects for level of treatment (placebo or disulfiram), the time point of the measure after giving dextroamphetamine (for cognitive and endocrine outcomes), and the interaction of these two effects. Also included were a random effect for participant and a blocking factor for treatment sequence. On the SART, the primary outcome measure was the number of errors of commission (out of 25) pressing the spacebar when “3” was displayed. The number of errors of omission (not pressing the spacebar on digits other than 3), and the mean correct reaction times to those digits were also measured, with reaction times quicker than 100 ms discarded from analysis. Due to computer error, SART data from two sessions were lost.
For physiological and subjective scales, where multiple measurements were taken at different time points, change-from-baseline scores were used as outcome measures. These scores, which are calculated using the maximum post-dose effect and the pre-dose baseline, are summary measures that capture the magnitude of the response. A significance level of p<0.05 was used for all analyses.
Results
Physiological Responses
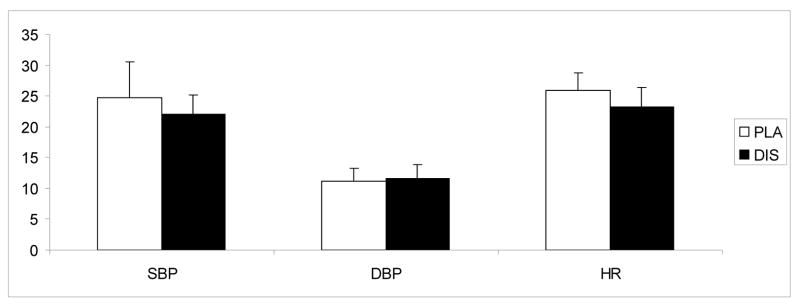
No significant treatment or treatment-by-time interaction was observed for dextroamphetamine-induced heart rate and blood pressure responses (Figure 1). There was no treatment effect on heart rate, systolic or diastolic blood pressure during the outpatient phase of the study.
Fig. 1.
The average (with standard error of the mean − SEM) systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate (HR) responses to 20 mg dextroamphetamine under 250 mg/day disulfiram (DIS) or placebo (PLA) conditions. Bars represent the change (maximum post dose/baseline). Measurements were taken at baseline, and then at 30, 60, 90, 120, 150, and 180 minutes after dextroamphetamine administration.
Subjective Responses
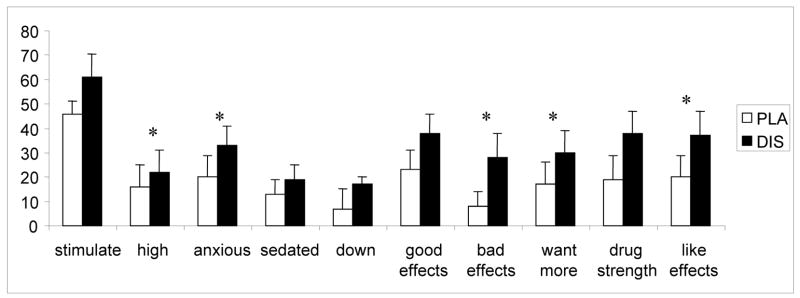
In response to dextroamphetamine, a significant main effect for treatment was observed on the DEQ for the rating of “high” [F (1, 8) = 6.2; p<0.05], “anxious” [F (1, 8) = 11.6; p<0.01], “bad drug effects” [F (1, 8) = 10.0; p<0.05], “want more drug” [F (1, 8) = 9.2; p<0.05], and “like drug effects” [F (1, 8) = 8.0; p<0.05]. In all these items, greater ratings were observed under disulfiram treatment (Figure 2). There were no treatment effects on POMS or ARCI scores.
Fig. 2.
The average (SEM), subjective responses to 20 mg dextroamphetamine combined with 250 mg/day disulfiram (DIS) or placebo (PLA). Bars represent the change (maximum post dose/baseline). Measurements were taken at baseline, then at 30, 60, 90, 120, 150, and 180 minutes after dextroamphetamine administration. Significant (p<0.05) treatment differences are indicated by asterisks (*).
Endocrine Responses
There was no significant treatment or treatment-by-time interaction for cortisol or prolactin responses (Figures 3 and 4).
Fig. 3.
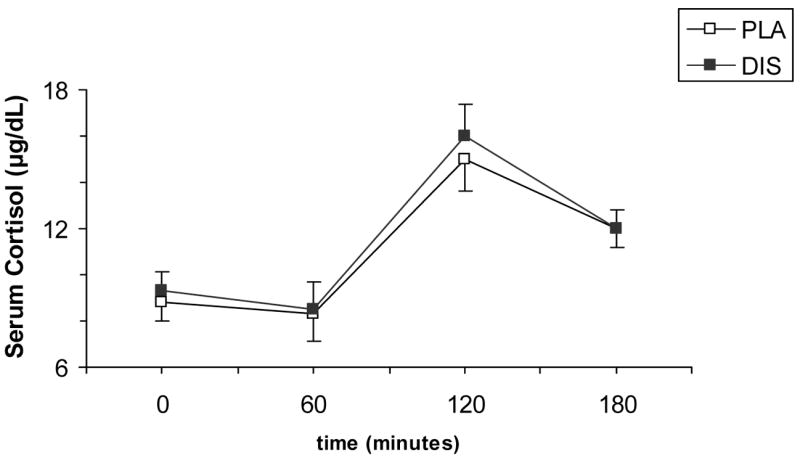
Average (SEM) plasma cortisol (μg/dL) responses to 20 mg dextroamphetamine combined with 250 mg/day disulfiram (DIS) or placebo (PLA). Measurements were taken at baseline, then at 60, 120, and 180 minutes after dextroamphetamine administration.
Fig. 4.
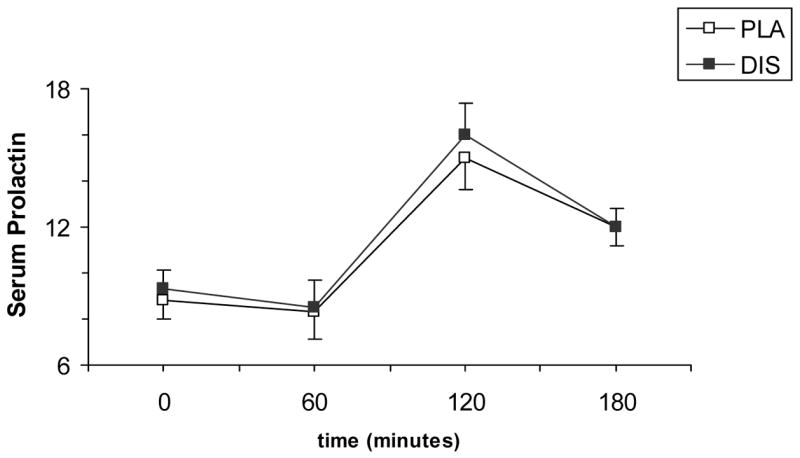
Average (SEM) plasma prolactin responses to 20 mg dextroamphetamine combined with 250 mg/day disulfiram (DIS) or placebo (PLA). Measurements were taken at baseline, and then at 60, 120, and 180 minutes after dextroamphetamine administration.
Cognitive Performance (SART)
More errors of omission occurred under disulfiram than placebo [F (1, 8.09) = 8.32; p=<.05]. There was also a trend for more errors of commission occurred under disulfiram than placebo [F (1, 7.51) = 6.2; p=0.055] (Figure 5). There was no significant effect of treatment on reaction times.
Fig 5.
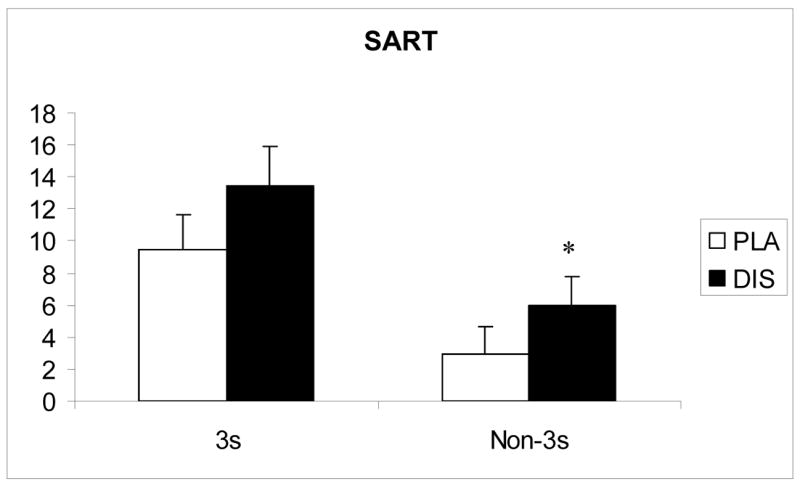
Number of errors on the SART following 20 mg dextroamphetamine combined with 250 mg/day disulfiram (DIS) or placebo (PLA). Significant (p<0.05) treatment differences are indicated by an asterisk (*)
Discussion
Disulfiram enhanced both the rewarding (e.g., drug liking) and aversive subjective effects of dextroamphetamine. Previous research involving disulfiram and cocaine found that disulfiram increased the rating of “nervousness” and “paranoia” from intranasal cocaine, without affecting the rating of “high” (Hameedi et al., 1995; McCance-Katz et al., 1998). In a more recent study, Baker et al. (2007) reported that disulfiram treatment attenuated the “high” from intravenous cocaine but enhanced the rating of “anxious”, suggesting that disulfiram’s efficacy in reducing cocaine use may be due its attenuation of the cocaine-induced euphoria and/or enhancement of cocaine-induced aversive subjective effects. In both studies, disulfiram administration resulted in significantly higher plasma cocaine levels, likely due to the inhibition of cocaine metabolism by disulfiram (Baker et al., 2007). Whether disulfiram affects amphetamine pharmacokinetics has not been examined and remains as a possible contributor to explain our findings. Another possibility is that disulfiram, through inhibition of the enzyme DBH, may increase the amount of dopamine in the brain by blocking dopamine’s conversion to norepinephrine and thereby increase the dopamine that amphetamine can release (Karamanakos et al., 2001).
It is not clear why disulfiram affects cocaine and amphetamine-induced subjective responses differently. We speculate that, while cocaine depends on simple reuptake blockade, the active release of dopamine from noradrenergic neurons terminating on the nucleus accumbens would likely increase amphetamine’s positive and negative subjective effects, as observed. Furthermore, dextroamphetamine and methamphetamine were five to nine times more potent than cocaine at the noradrenergic transporter than the dopamine transporter (Han and Gu, 2006), thereby again producing a significantly greater surge of dopamine from these noradrenergic neurons that are now releasing dopamine after disulfiram treatment. From the treatment perspective, how these disulfiram-induced increases in both positive and negative subjective responses to amphetamine affect drug use behavior remains to be determined in future studies.
In our study, disulfiram did not enhance the heart rate and blood pressure increases induced by dextroamphetamine, suggesting that the combination of disulfiram and dextroamphetamine is no less safe than dextroamphetamine alone. Although a previous study combining disulfiram with intranasal cocaine reported modest enhancement of cocaine-induced heart rate and blood pressure increases (McCance-Katz et al., 1998), intravenous cocaine, which is closer to the potency of amphetamine by any route of administration, did not enhance the cocaine-induced blood pressure and heart rate increases (Baker et al., 2007). Thus, the combination of disulfiram and amphetamine appears medically safe.
Disulfiram combined with dextroamphetamine was associated with more errors on the SART than the placebo-dextroamphetamine combination. Because disulfiram’s effects on cognitive performance have not been well-described, these findings warrant further examination of disulfiram on cognitive performance. The treatment relevance of this cognitive impairment is difficult to predict, since poor cognition could interfere with cognitive behavioral therapy interventions, but a lack of cognitive enhancement by amphetamine when co-administered with disfulfiram could remove another rewarding aspect of amphetamine abuse and thereby help reduce amphetamine abuse.
As expected, both cortisol and prolactin levels were sensitive to dextroamphetamine. Although the hypothalamic-pituitary-adrenal (HPA) axis including cortisol secretion is enhance by norepinephrine (Charmandari et al., 2005) and prolactin secretion is inhibited by dopamine (Freeman et al., 2000), disulfiram’s potential to reduce norepinephrine and increase dopamine did not enhance the dextroamphetamine-induced cortisol or prolactin increases. This lack of effect is probably due to the re-equilibration of these hormonal systems after 4 days of disulfiram induced changes and the relatively more potent acute amphetamine effect on these hormones. This lack of effect is also consistent with disulfiram’s lack of cardiovascular enhancements of amphetamine effects.
This study had several limitations. First, we did not examine the dose-dependent effects of disulfiram, since only a single 250 mg/day dose was used. Second, the study did not examine disulfiram’s effects on dose-dependent dextroamphetamine responses, since only a single 20 mg dose of dextroamphetamine was used. Third, there was no placebo control for the dextroamphetamine administration, since all subjects received dextroamphetamine on the fourth day of both treatment periods. Lastly, our subjects were healthy controls, so the generalization of these findings to stimulant users still needs to be demonstrated.
In summary, disulfiram 250 mg/day enhanced some of the subjective effects of dextroamphetamine without affecting the cardiovascular and endocrine responses. Further studies are warranted to examine the therapeutic utility of disulfiram for amphetamine addiction.
Acknowledgments
This research was supported by the Veterans Administration Mental Illness Research, Education and Clinical Center (MIRECC) and National Institute on Drug Abuse (NIDA) grants P50-DA12762, KO2 DA021304 (MS)and K05-DA0454 (TRK). We would like to thank Ellen Mitchell, R.N., Lance Barnes, and Stacy Minnix for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker JR, Jatlow P, McCance-Katz EF. Disulfiram effects on responses to intravenous cocaine administration. Drug Alcohol Depend. 2007;87:202–209. doi: 10.1016/j.drugalcdep.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer LH, de Wit H. Subjective responses to d-amphetamine alone and after pimozide pretreatment in normal, healthy volunteers. Biol Psychiatry. 1996;39:26–32. doi: 10.1016/0006-3223(95)00110-7. [DOI] [PubMed] [Google Scholar]
- Carroll K, Ziedonis D, O'Malley S, et al. Pharmacologic interventions for alcohol- and cocaine-abusing individuals: a pilot study of disulfiram vs. naltrexone. American Journal on Addictions. 1993;2:77–79. [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2004;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93:713–727. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Zabetian CP. Human genetics of plasma dopamine beta-hydroxylase activity: applications to research in psychiatry and neurology. Psychopharmacology (Berl) 2004;174:463–476. doi: 10.1007/s00213-004-1840-8. [DOI] [PubMed] [Google Scholar]
- Faiman MD, Jensen JC, Lacoursiere RB. Elimination kinetics of disulfiram in alcoholics after single and repeated doses. Clin Pharmacol Ther. 1984;36:520–526. doi: 10.1038/clpt.1984.213. [DOI] [PubMed] [Google Scholar]
- Farrin L, Hull L, Unwin C, Wykes T, David A. Effects of depressed mood on objective and subjective measures of attention. J Neuropsychiatry Clin Neurosci. 2003;15:98–104. doi: 10.1176/jnp.15.1.98. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, Marczinski CA. Effects of d-amphetamine on behavioral control in stimulant abusers: the role of prepotent response tendencies. Drug Alcohol Depend. 2003;71:143–152. doi: 10.1016/s0376-8716(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR, Resnekov L, Shick JF, Krasnegor NA, Fennell W, Freedman DX. Cardiovascular and subjective effects of intravenous cocaine administration in humans. Arch Gen Psychiatry. 1976;33:983–989. doi: 10.1001/archpsyc.1976.01770080101010. [DOI] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- George TP, Chawarski MC, Pakes J, Carroll KM, Kosten TR, Schottenfeld RS. Disulfiram versus placebo for cocaine dependence in buprenorphine-maintained subjects: a preliminary trial. Biol Psychiatry. 2000;47:1080–1086. doi: 10.1016/s0006-3223(99)00310-8. [DOI] [PubMed] [Google Scholar]
- Grady TA, Broocks A, Canter SK, Pigott TA, Dubbert B, Hill JL, Murphy DL. Biological and behavioral responses to D-amphetamine, alone and in combination with the serotonin3 receptor antagonist ondansetron, in healthy volunteers. Psychiatry Res. 1996;64:1–10. doi: 10.1016/0165-1781(96)02884-3. [DOI] [PubMed] [Google Scholar]
- Hameedi FA, Rosen MI, McCance-Katz EF, McMahon TJ, Price LH, Jatlow PI, Woods SW, Kosten TR. Behavioral, physiological, and pharmacological interaction of cocaine and disulfiram in humans. Biol Psychiatry. 1995;37:560–563. doi: 10.1016/0006-3223(94)00361-6. [DOI] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KP, Sofuoglu M. Biological treatments for amfetamine dependence : recent progress. CNS Drugs. 2007;21:851–869. doi: 10.2165/00023210-200721100-00005. [DOI] [PubMed] [Google Scholar]
- Hughes JC, Cook CC. The efficacy of disulfiram: a review of outcome studies. Addiction. 1997;92:381–395. [PubMed] [Google Scholar]
- Karamanakos PN, Pappas P, Stephanou P, Marselos M. Differentiation of disulfiram effects on central catecholamines and hepatic ethanol metabolism. Pharmacol Toxicol. 2001;88:106–110. [PubMed] [Google Scholar]
- Lott DC, Kim SJ, Cook EH, de Wit H. Dopamine transporter gene associated with diminished subjective response to amphetamine. Neuropsychopharmacology. 2005;30:602–609. doi: 10.1038/sj.npp.1300637. [DOI] [PubMed] [Google Scholar]
- Manly T, Davison B, Heutink J, Galloway M, Robertson IH. Not enough time or not enough attention? Speed, error and self-maintained control in the Sustained Attention to Response Test (SART) Clinical Neuropsychological Assessment. 2000;3:167–177. [Google Scholar]
- Manly T, Lewis GH, Robertson IH, Watson PC, Datta AK. Coffee in the cornflakes: time-of-day as a modulator of executive response control. Neuropsychologia. 2002;40:1–6. doi: 10.1016/s0028-3932(01)00086-0. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Disulfiram effects on acute cocaine administration. Drug Alcohol Depend. 1998;52:27–39. doi: 10.1016/s0376-8716(98)00050-7. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Dropperman L. Manual for profile of mood states. San Diego, CA: Educational and industrial testing services; 1971. [Google Scholar]
- Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T, Rounsaville BJ. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction. 2000;95:219–228. doi: 10.1046/j.1360-0443.2000.9522198.x. [DOI] [PubMed] [Google Scholar]
- Physicians Desk Reference. Montvale, N.J.: Medical Economics Data; 2008. [Google Scholar]
- Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. 'Oops!': performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia. 1997;35:747–758. doi: 10.1016/s0028-3932(97)00015-8. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2003 National Survey on Drug Use and Health: national findings. 2004. Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- Shallice T, Marzocchi GM, Coser S, Del Savio M, Meuter RF, Rumiati RI. Executive function profile of children with attention deficit hyperactivity disorder. Dev Neuropsychol. 2002;21:43–71. doi: 10.1207/S15326942DN2101_3. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Kosten TR. Emerging pharmacological strategies in the fight against cocaine addiction. Expert Opin Emerg Drugs. 2006;11:91–98. doi: 10.1517/14728214.11.1.91. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Mooney M, Kosten T. Riluzole and d-amphetamine interactions in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:16–22. doi: 10.1016/j.pnpbp.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, O'Neill SC, Higgins ST. d-amphetamine increases choice of cigarette smoking over monetary reinforcement. Psychopharmacology (Berl) 2000;153:85–92. doi: 10.1007/s002130000600. [DOI] [PubMed] [Google Scholar]
- Vaccari A, Saba PL, Ruiu S, Collu M, Devoto P. Disulfiram and diethyldithiocarbamate intoxication affects the storage and release of striatal dopamine. Toxicol Appl Pharmacol. 1996;139:102–108. doi: 10.1006/taap.1996.0147. [DOI] [PubMed] [Google Scholar]