Abstract
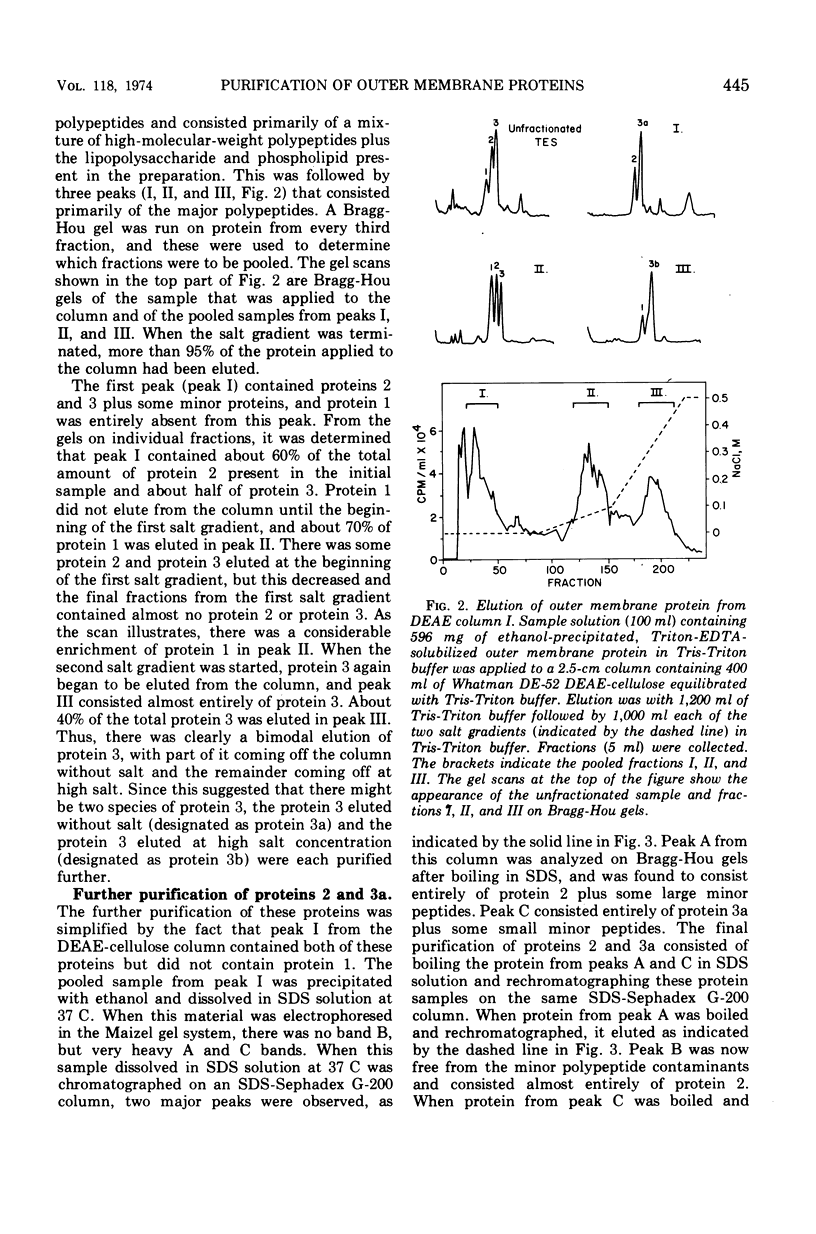
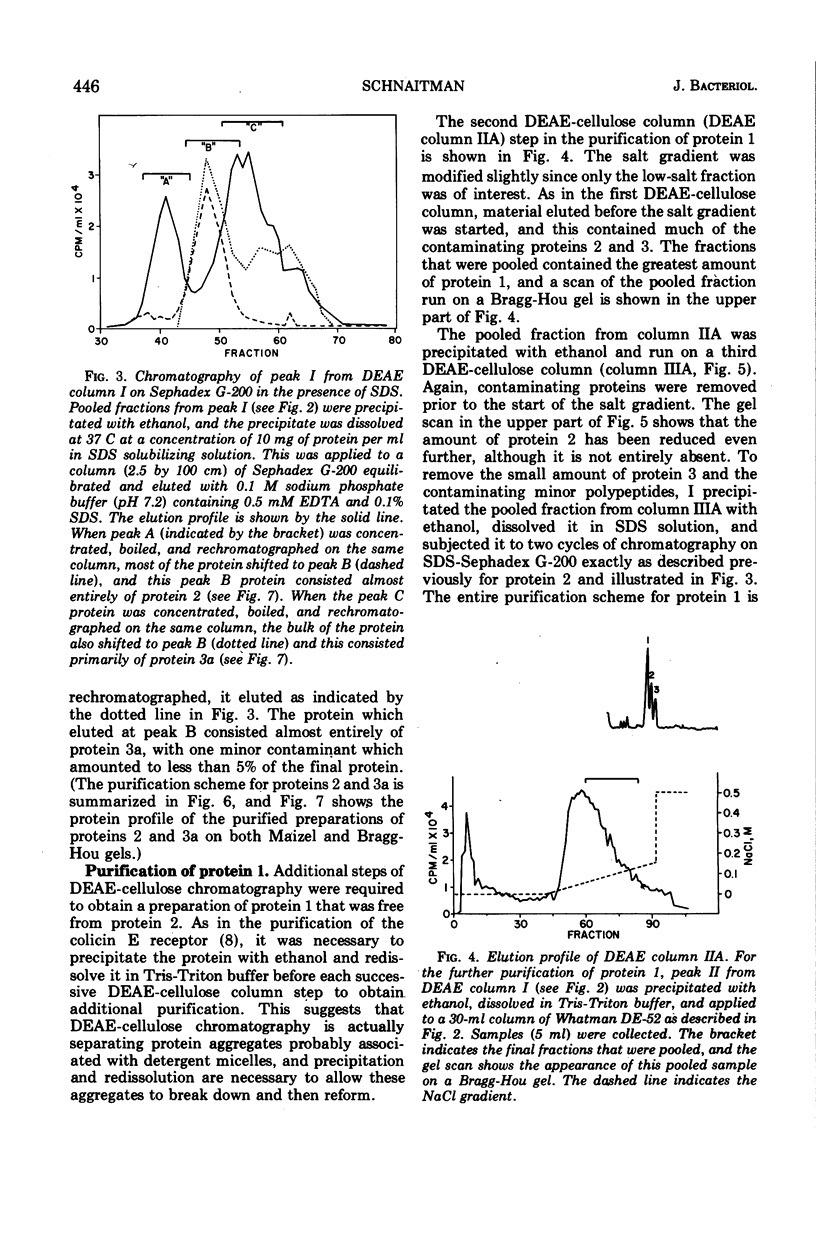
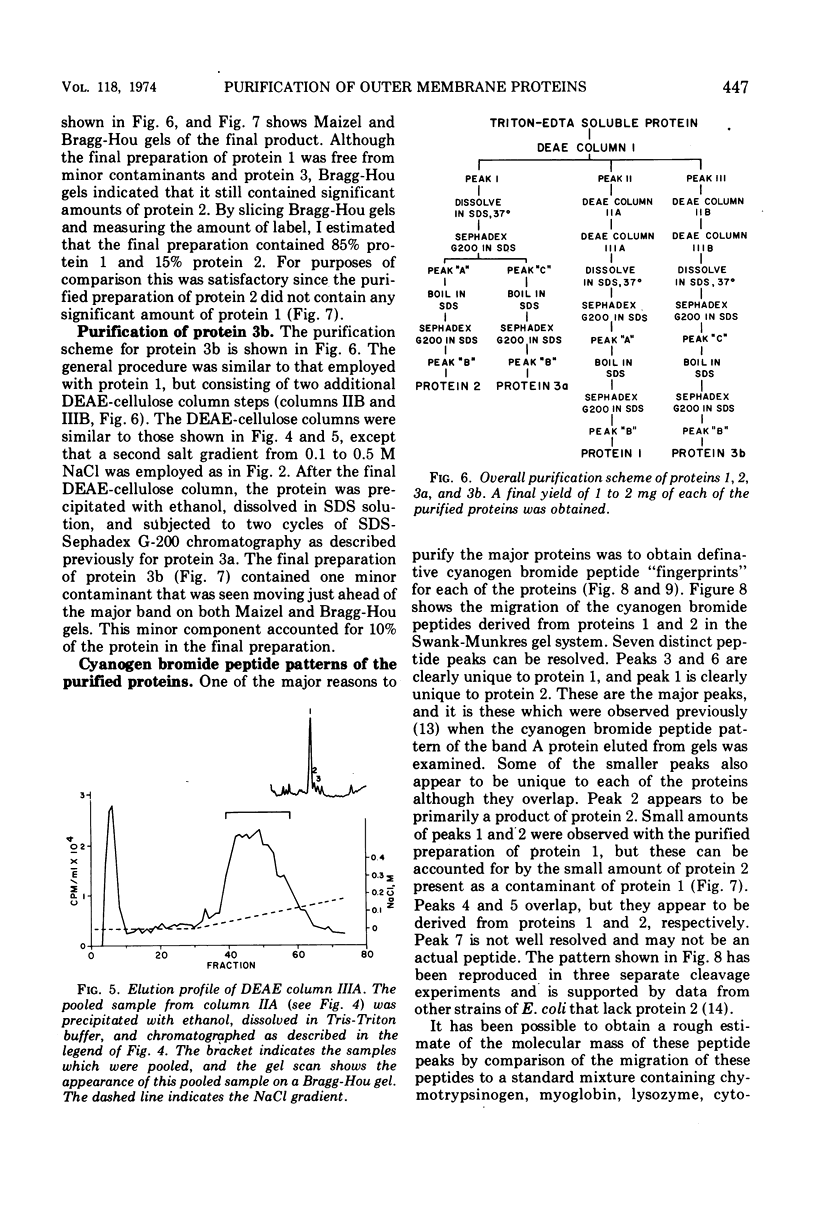
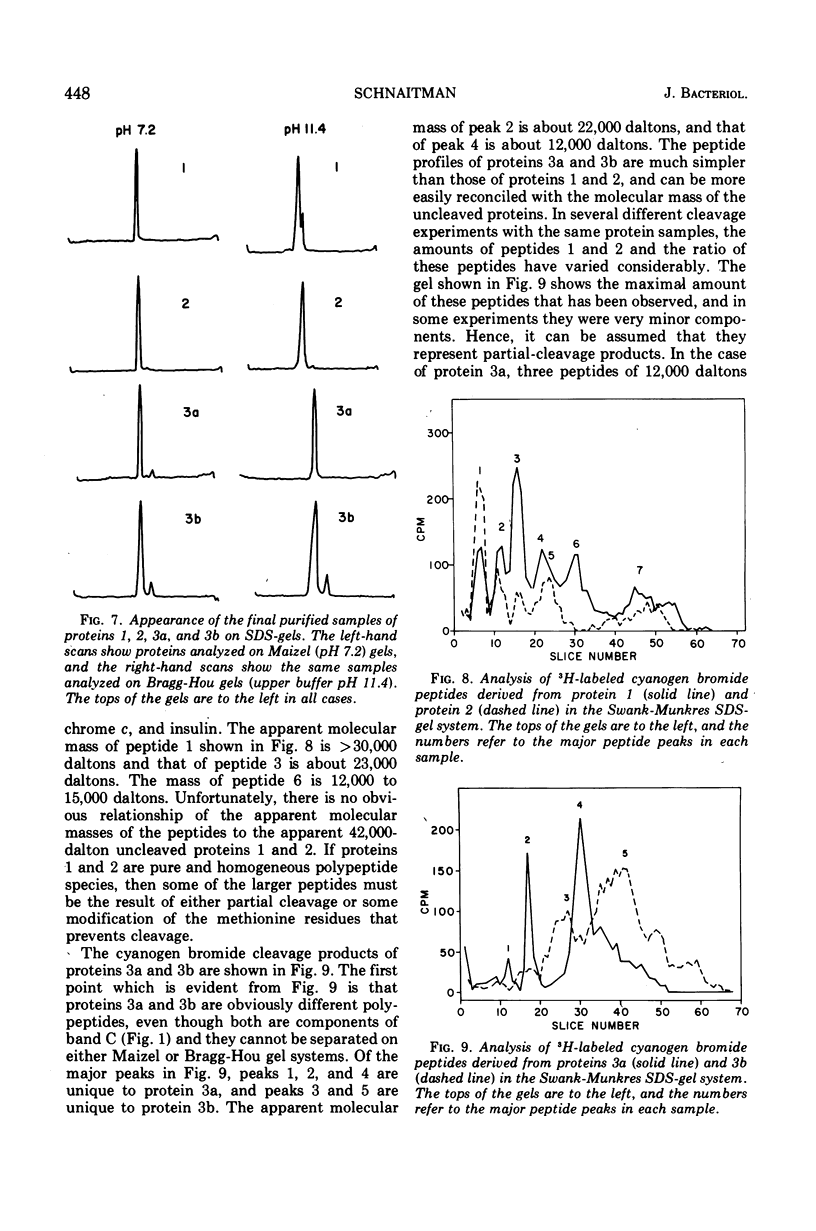
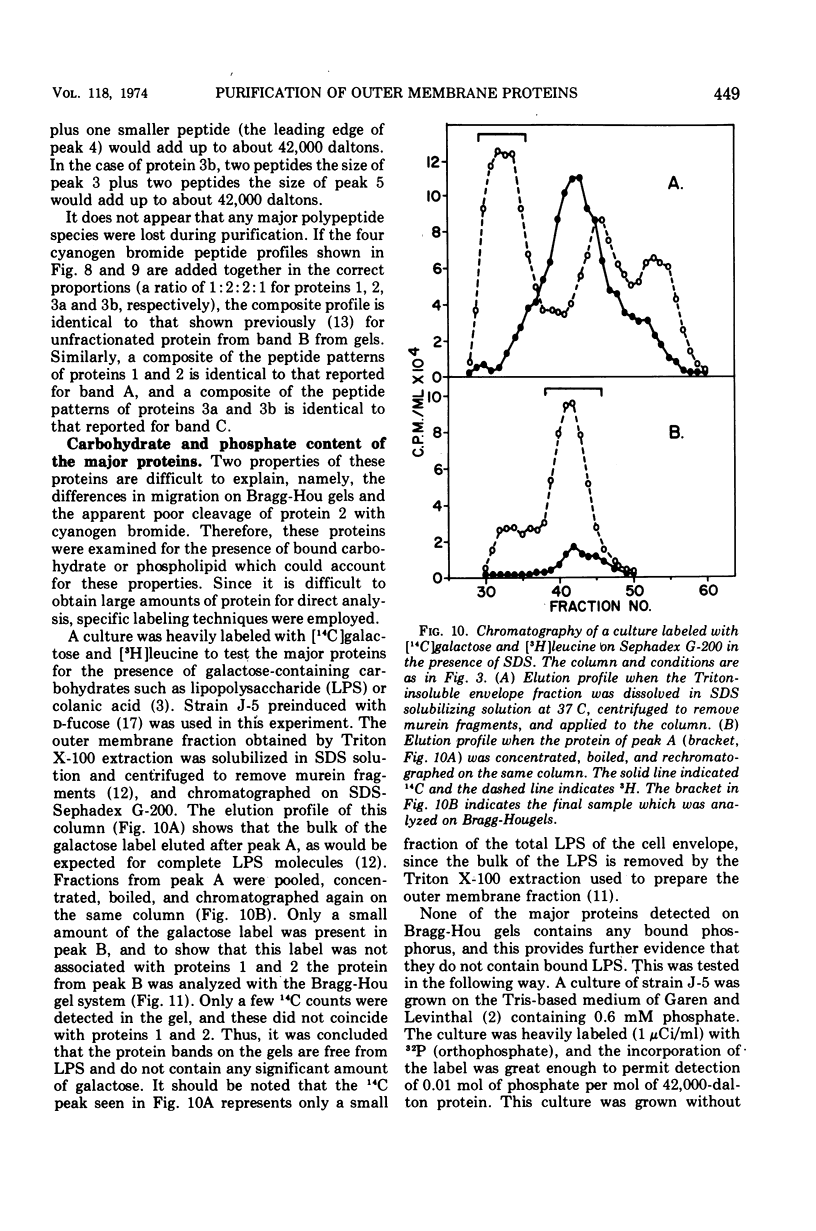
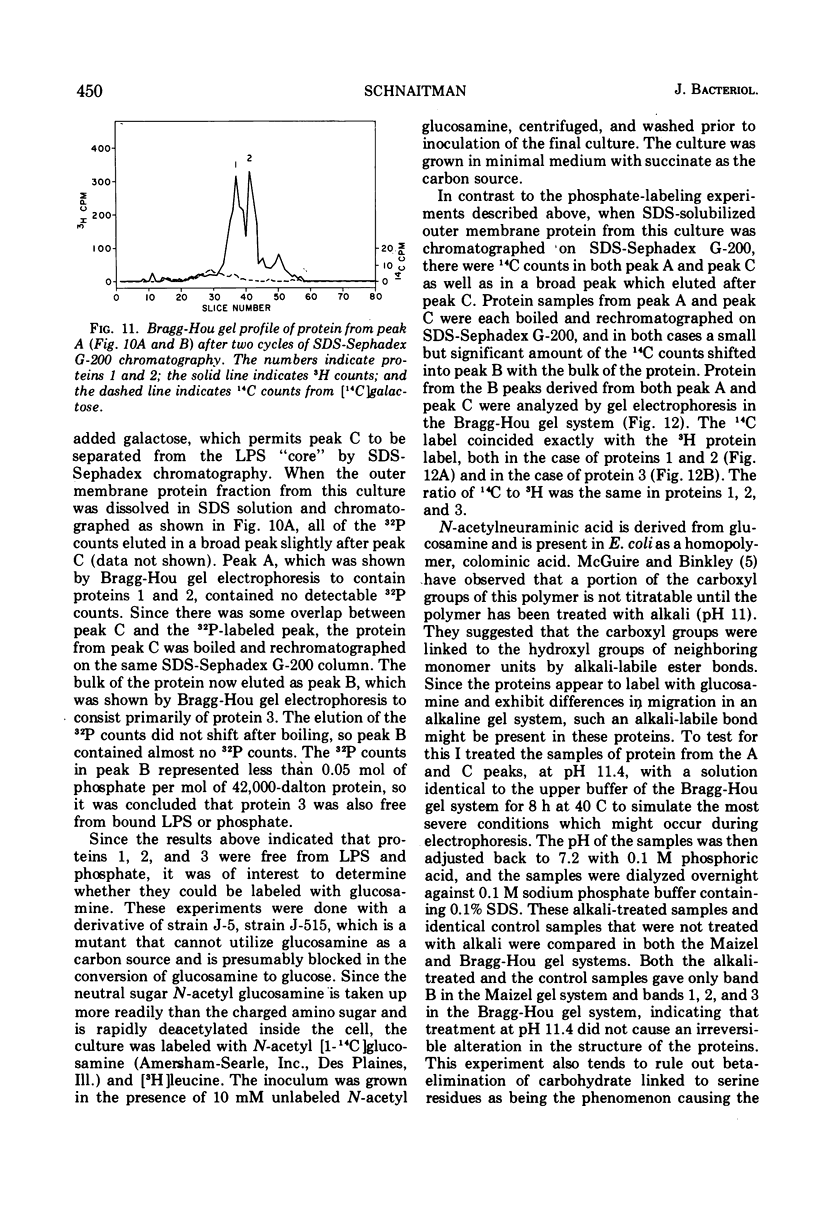
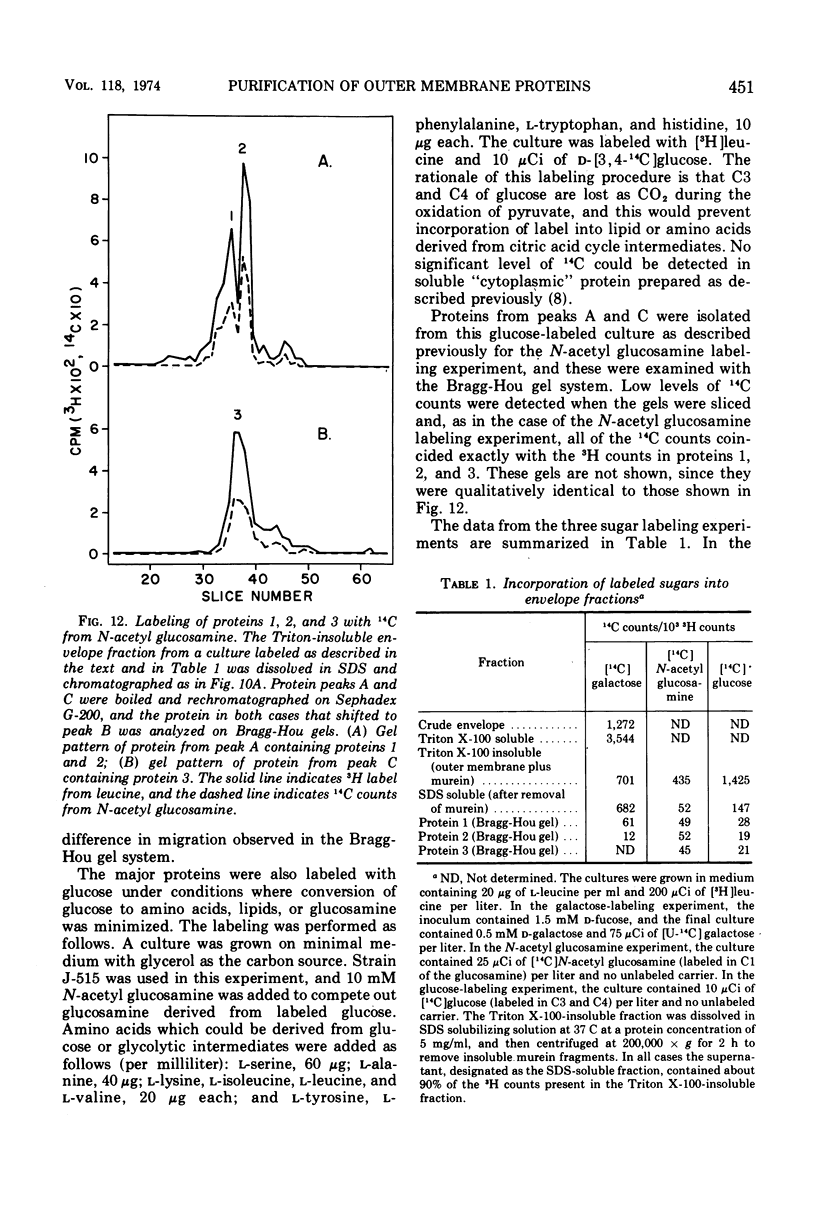
Previous studies have shown that the outer membrane of Escherichia coli O111 gives a single, major, 42,000-dalton protein peak when analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis at neutral pH. Further studies have shown that this peak consists of more than a single polypeptide species, and on alkaline SDS-gel electrophoresis this single peak is resolved into three subcomponents designated as proteins 1, 2, and 3. By chromatography of solubilized, outer membrane protein on diethylaminoethyl-cellulose followed by chromatography on Sephadex G-200 in the presence of SDS, it was possible to separate the 42,000-dalton major protein into four distinct protein fractions. Comparison of cyanogen bromide peptides derived from these fractions indicated that they represented at least four distinct polypeptide species. Two of these proteins migrated as proteins 1 and 2 on alkaline gels. The other two proteins migrated as protein 3 on alkaline gels and cannot be separated by SDS-polyacrylamide gel electrophoresis. In purified form, these major proteins do not contain bound lipopolysaccharide, phospholipid, or phosphate. These proteins may contain a small amount of carbohydrate, as evidenced by the labeling of these proteins by glucosamine, and to a lesser extent by glucose, under conditions where the metabolism of these sugars to amino acids and lipids is blocked. All of the proteins were labeled to the same extent by these sugars. Thus, it was concluded that there are at least four distinct polypeptide species with apparent molecular masses of about 42,000 daltons in the outer membrane of E. coli O111.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bragg P. D., Hou C. Organization of proteins in the native and reformed outer membrane of Escherichia coli. Biochim Biophys Acta. 1972 Aug 9;274(2):478–488. doi: 10.1016/0005-2736(72)90193-9. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- GOEBEL W. F. Colanic acid. Proc Natl Acad Sci U S A. 1963 Apr;49:464–471. doi: 10.1073/pnas.49.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Yee M. L. Homogeneity of envelope proteins of Escherichia coli separated by gel electrophoresis in sodium dodecyl sulfate. J Bacteriol. 1973 Jan;113(1):304–312. doi: 10.1128/jb.113.1.304-312.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGUIRE E. J., BINKLEY S. B. THE STRUCTURE AND CHEMISTRY OF COLOMINIC ACID. Biochemistry. 1964 Feb;3:247–251. doi: 10.1021/bi00890a017. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- Moldow C., Robertson J., Rothfield L. Purification of bacterial membrane proteins. The use of guanidinium thiocyanate and urea. J Membr Biol. 1972;10(2):137–152. doi: 10.1007/BF01867850. [DOI] [PubMed] [Google Scholar]
- Sabet S. F., Schnaitman C. A. Purification and properties of the colicin E3 receptor of Escherichia coli. J Biol Chem. 1973 Mar 10;248(5):1797–1806. [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. I. Effect of preparative conditions on the migration of protein in polyacrylamide gels. Arch Biochem Biophys. 1973 Aug;157(2):541–552. doi: 10.1016/0003-9861(73)90673-5. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. II. Heterogeneity of major outer membrane polypeptides. Arch Biochem Biophys. 1973 Aug;157(2):553–560. doi: 10.1016/0003-9861(73)90674-7. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. IV. Differences in outer membrane proteins due to strain and cultural differences. J Bacteriol. 1974 May;118(2):454–464. doi: 10.1128/jb.118.2.454-464.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- White D. A., Lennarz W. J., Schnaitman C. A. Distribution of lipids in the wall and cytoplasmic membrane subfractions of the cell envelope of Escherichia coli. J Bacteriol. 1972 Feb;109(2):686–690. doi: 10.1128/jb.109.2.686-690.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C. Isolation and characterization of an Escherichia coli mutant with alteration in the outer membrane porteins of the cell envelope. Biochim Biophys Acta. 1972 Dec 1;290(1):274–289. doi: 10.1016/0005-2736(72)90070-3. [DOI] [PubMed] [Google Scholar]
- Wu M. C., Heath E. C. Isolation and characterization of lipopolysaccharide protein from Escherichia coli. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2572–2576. doi: 10.1073/pnas.70.9.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]


