Abstract
Bioluminescent marine bacteria possess luciferase, which catalyzes the oxidation of reduced flavin mononucleotide and long-chain aldehyde to produce light. Temperature-sensitive mutants of these bacteria can be obtained which require exogenous aldehyde for light production at higher temperatures. In Beneckea harveyi. two classes of such mutants were found which differed with regard to their response to temperature shifts. In one class, a shift from permissive to nonpermissive temperature in liquid cultures resulted in a rapid (t½ ≃ 3 min) loss of luminescence. In the other, there was no immediate decline in luminescence; it was the increase of luminescence that was blocked. Through studies of these and other effects of temperature shifts on the in vivo luminescence of these mutants, we conclude that at least two genes are specifically involved in the in vivo biosynthesis of aldehyde for the luminescence reaction and that both genes are coordinately controlled with that for luciferase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley D., Eberhard A., Solsky R. Decyl nitrite: an aldehyde analog in the bacterial bioluminescence reaction. Biochem Biophys Res Commun. 1974 Feb 27;56(4):865–868. doi: 10.1016/s0006-291x(74)80268-8. [DOI] [PubMed] [Google Scholar]
- Cline T. W., Hastings J. W. Mutationally altered bacterial luciferase. Implications for subunit functions. Biochemistry. 1972 Aug 29;11(18):3359–3370. doi: 10.1021/bi00768a008. [DOI] [PubMed] [Google Scholar]
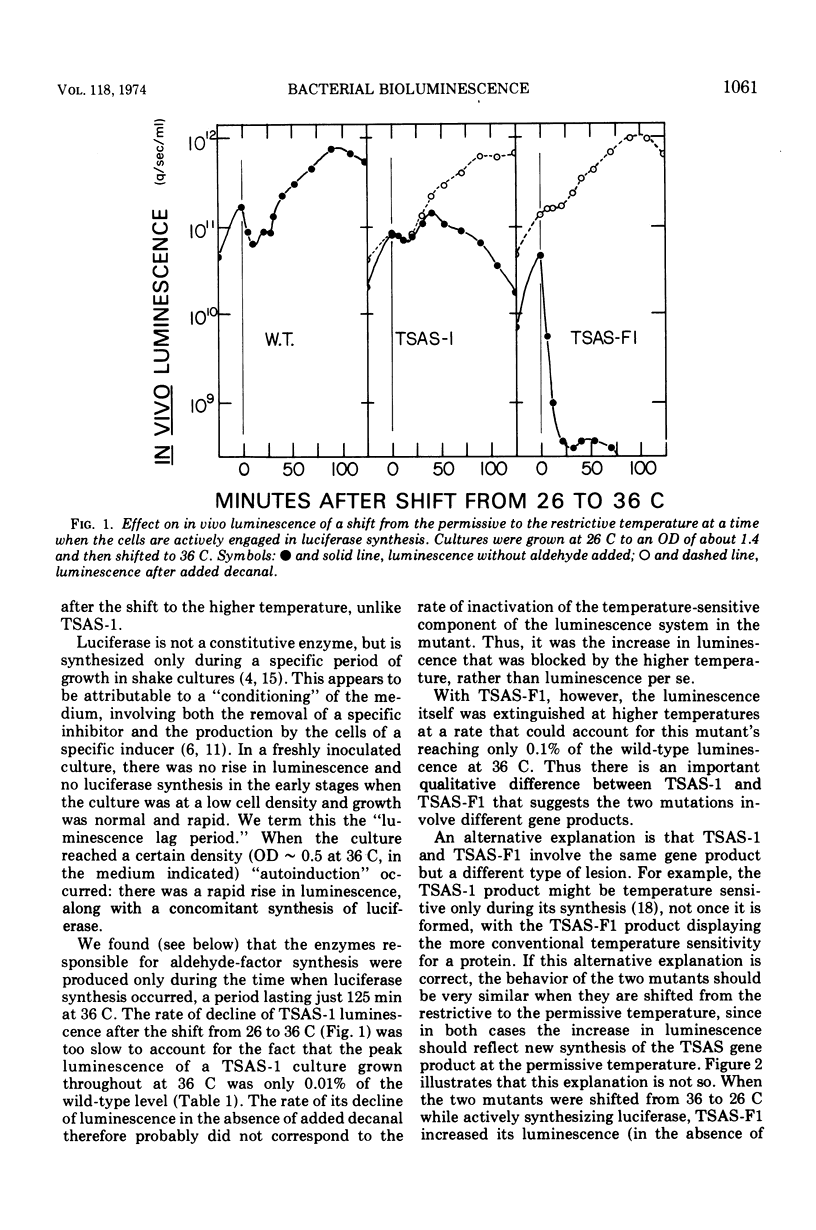
- Cline T., Hastings J. W. Temperature-sensitive mutants of bioluminescent bacteria. Proc Natl Acad Sci U S A. 1971 Feb;68(2):500–504. doi: 10.1073/pnas.68.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey J. J. Inducible synthesis of bacterial luciferase: specificity and kinetics of induction. J Bacteriol. 1967 Nov;94(5):1638–1647. doi: 10.1128/jb.94.5.1638-1647.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn D. K., Michaliszyn G. A., Bogacki I. G., Meighen E. A. Conversion of aldehyde to acid in the bacterial bioluminescent reaction. Biochemistry. 1973 Nov 20;12(24):4911–4918. doi: 10.1021/bi00748a016. [DOI] [PubMed] [Google Scholar]
- Eberhard A. Inhibition and activation of bacterial luciferase synthesis. J Bacteriol. 1972 Mar;109(3):1101–1105. doi: 10.1128/jb.109.3.1101-1105.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell W. J., Kessler R. J., Drouillard M. Identification of n-nonaldehyde in Photobacterium fisheri. Chem Phys Lipids. 1971 May;6(2):131–134. doi: 10.1016/0009-3084(71)90036-3. [DOI] [PubMed] [Google Scholar]
- Hastings J. W., Weber K., Friedland J., Eberhard A., Mitchell G. W., Gunsalus A. Structurally distinct bacterial luciferases. Biochemistry. 1969 Dec;8(12):4681–4689. doi: 10.1021/bi00840a004. [DOI] [PubMed] [Google Scholar]
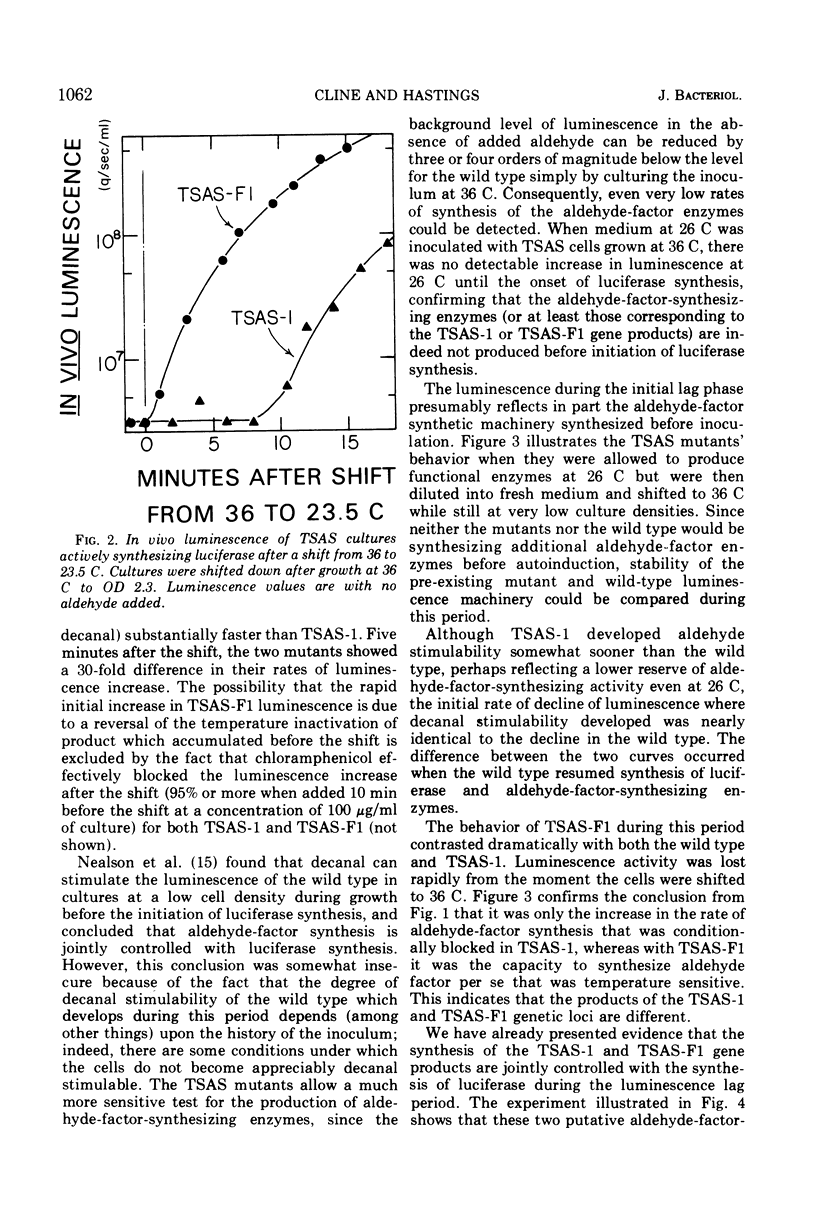
- Kempner E. S., Hanson F. E. Aspects of light production by Photobacterium fischeri. J Bacteriol. 1968 Mar;95(3):975–979. doi: 10.1128/jb.95.3.975-979.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCapra F., Hysert D. W. Bacterial bioluminescence-identification of fatty acid as product, its quantum yield and a suggested mechanism. Biochem Biophys Res Commun. 1973 May 1;52(1):298–304. doi: 10.1016/0006-291x(73)90987-x. [DOI] [PubMed] [Google Scholar]
- Mitchell G. W., Hastings J. W. A stable, inexpensive, solid-state photomultiplier photometer. Anal Biochem. 1971 Jan;39(1):243–250. doi: 10.1016/0003-2697(71)90481-7. [DOI] [PubMed] [Google Scholar]
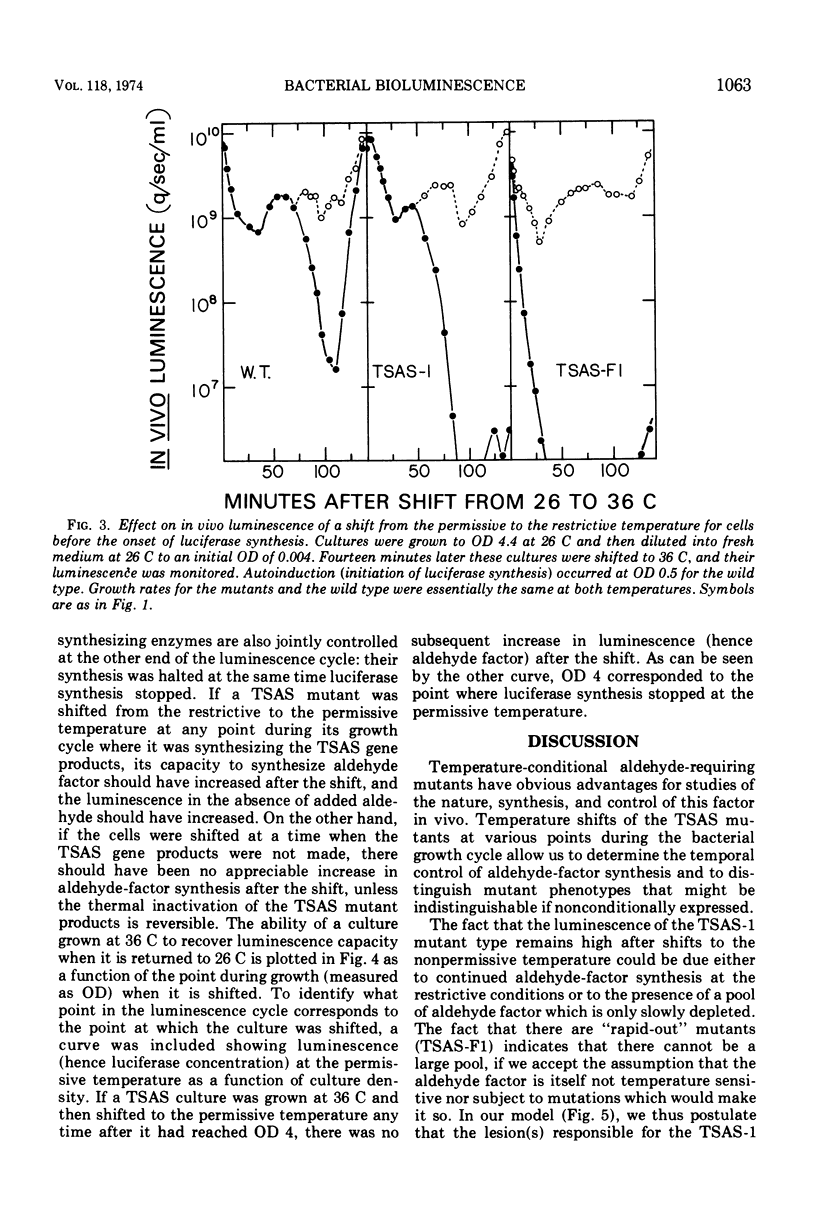
- Nealson K. H., Markovitz A. Mutant analysis and enzyme subunit complementation in bacterial bioluminescence in Photobacterium fischeri. J Bacteriol. 1970 Oct;104(1):300–312. doi: 10.1128/jb.104.1.300-312.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H., Platt T., Hastings J. W. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970 Oct;104(1):313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers P., McElroy W. D. BIOCHEMICAL CHARACTERISTICS OF ALDEHYDE AND LUCIFERASE MUTANTS OF LUMINOUS BACTERIA. Proc Natl Acad Sci U S A. 1955 Feb 15;41(2):67–70. doi: 10.1073/pnas.41.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodman S. T., Greenberg G. R. Conditions allowing synthesis of thymidylate synthetase at the nonpermissive temperature in a temperature-sensitive thy mutant normally blocked in its translation. J Biol Chem. 1971 Aug 10;246(15):4853–4858. [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H., Kohama Y. Reactions involved in bioluminescence systems of limpet (Latia neritoides) and luminous bacteria. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2086–2089. doi: 10.1073/pnas.69.8.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]


