Abstract
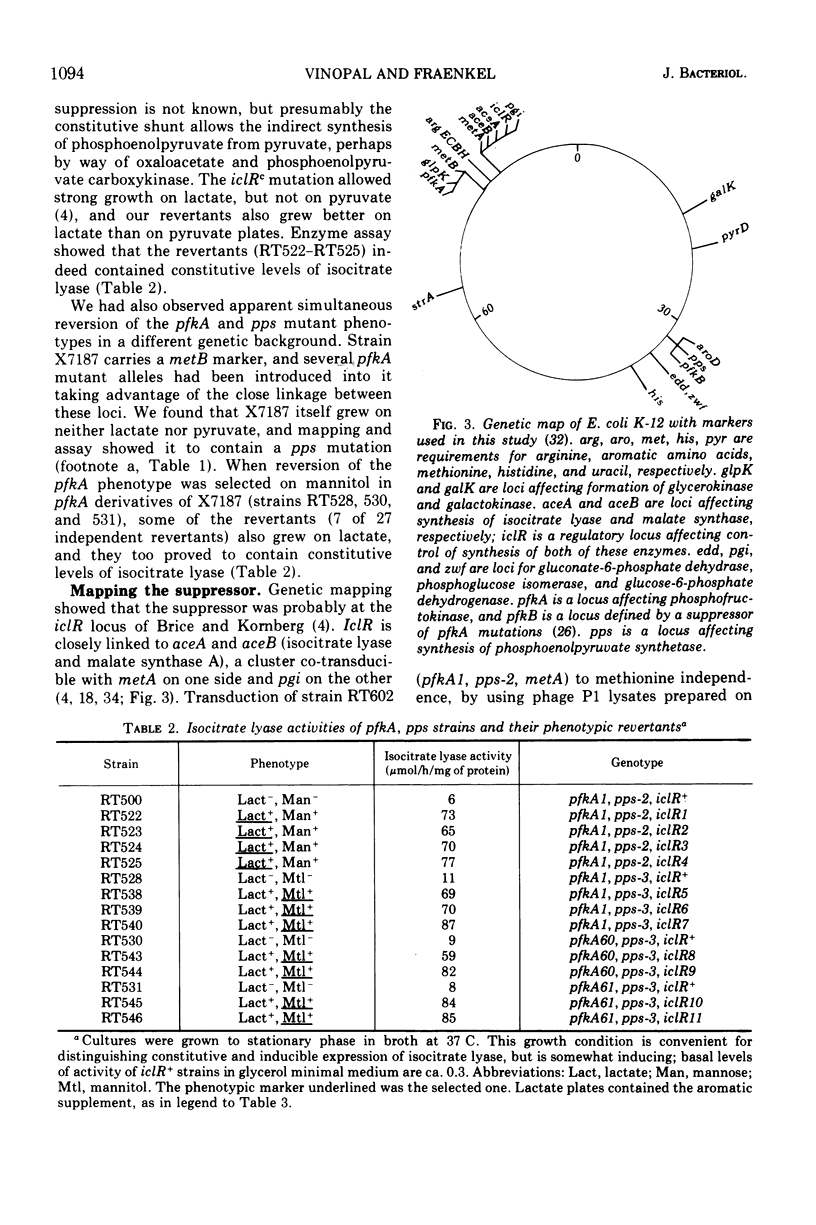
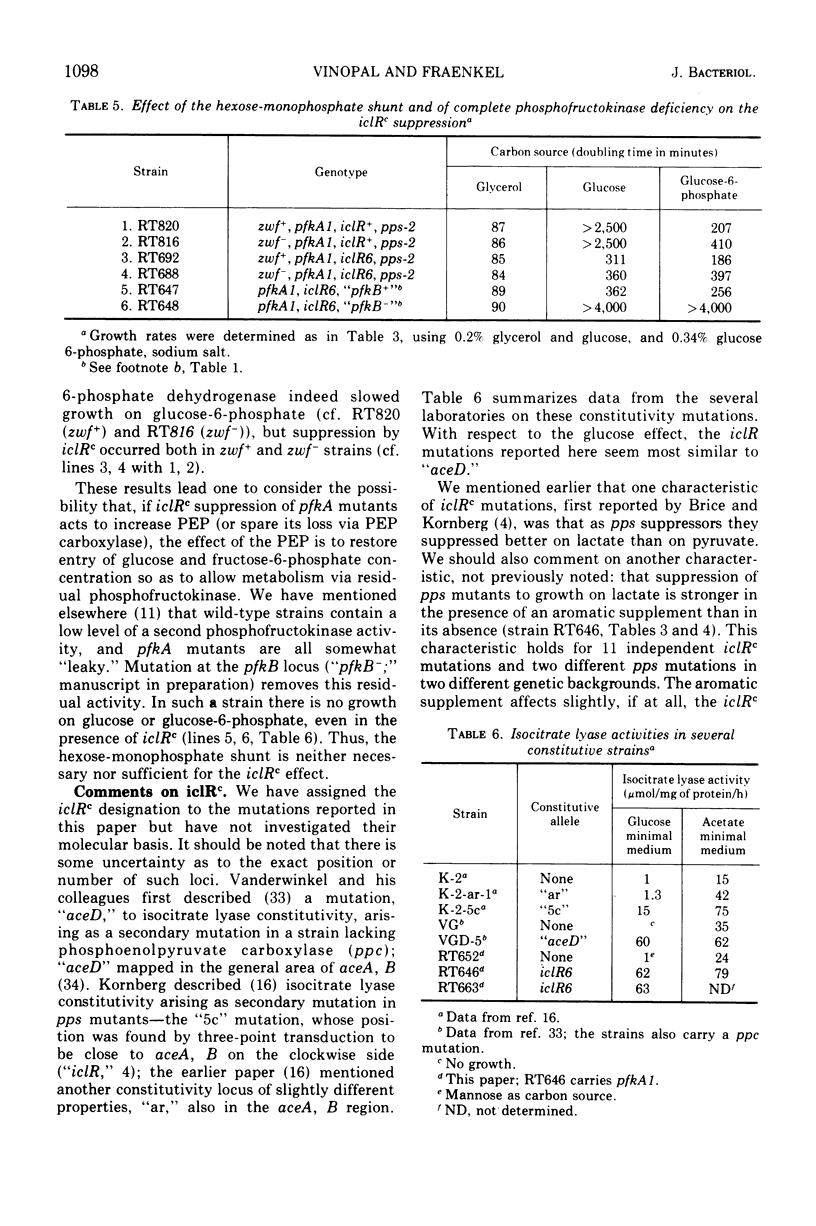
Fructose-6-phosphate kinase (pfkA) mutants have impaired growth on carbon sources which enter glycolysis at or above the level of fructose-6-phosphate, but the degree of impairment depends on the carbon source (e.g., growth on glucose is very much slower than growth on glucose-6-phosphate). The present report contains considerable data on this complicated growth phenotype and derives mainly from the finding of a class of partial revertants which grow as fast on glucose as on glucose-6-phosphate; the reversion mutation is shown to be constitutivity of the glyoxylate shunt (iclRc). iclRc does not increase the fructose-6-phosphate kinase level in the mutants, and the exact mechanism of the partial phenotypic suppression is not understood. However, iclRc was already known to suppress some mutations which affected phosphoenolpyruvate levels, and H. L. Kornberg and J. Smith have suggested (1970) that the growth phenotype of pfkA mutants might be related to pathways of phosphoenolpyruvate formation. Surprisingly, the hexose-monophosphate shunt is not necessary for the suppression, which therefore must act to restore metabolism via the residual phosphofructokinase activity present in all pfkA mutants. A mutant totally lacking phosphofructokinase activity was not suppressed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee S., Fraenkel D. G. Glucose-6-phosphate dehydrogenase from Escherichia coli and from a "high-level" mutant. J Bacteriol. 1972 Apr;110(1):155–160. doi: 10.1128/jb.110.1.155-160.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice C. B., Kornberg H. L. Genetic control of isocitrate lyase activity in Escherichia coli. J Bacteriol. 1968 Dec;96(6):2185–2186. doi: 10.1128/jb.96.6.2185-2186.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice C. B., Kornberg H. L. Location of a gene specifying phosphopyruvate synthase activity on the genome of Escherichia coli, K12. Proc R Soc Lond B Biol Sci. 1967 Sep 12;168(1012):281–292. doi: 10.1098/rspb.1967.0066. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Chulavatnatol M., Atkinson D. E. Phosphoenolpyruvate synthetase from Escherichia coli. Effects of adenylate energy charge and modifier concentrations. J Biol Chem. 1973 Apr 25;248(8):2712–2715. [PubMed] [Google Scholar]
- Epstein W., Jewett S., Fox C. F. Isolation and mapping of phosphotransferase mutants in Escherichia coli. J Bacteriol. 1970 Nov;104(2):793–797. doi: 10.1128/jb.104.2.793-797.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G. Genetic mapping of mutations affecting phosphoglucose isomerase and fructose diphosphatase in Escherichia coli. J Bacteriol. 1967 May;93(5):1582–1587. doi: 10.1128/jb.93.5.1582-1587.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G., Kotlarz D., Buc H. Two fructose 6-phosphate kinase activities in Escherichia coli. J Biol Chem. 1973 Jul 10;248(13):4865–4866. [PubMed] [Google Scholar]
- Fraenkel D. G., Levisohn S. R. Glucose and gluconate metabolism in an Escherichia coli mutant lacking phosphoglucose isomerase. J Bacteriol. 1967 May;93(5):1571–1578. doi: 10.1128/jb.93.5.1571-1578.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., KAUFMAN H. Selecting bacterial mutants by the penicillin method. Science. 1960 Feb 26;131(3400):604–605. doi: 10.1126/science.131.3400.604. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L., Smith J. Role of phosphofructokinase in the utilization of glucose by Escherichia coli. Nature. 1970 Jul 4;227(5253):44–46. doi: 10.1038/227044a0. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L., Smith J. Temperature-sensitive synthesis of isocitrate lyase in Escherichia coli. Biochim Biophys Acta. 1966 Sep;123(3):654–657. doi: 10.1016/0005-2787(66)90243-7. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L. The regulation of anaplerotic enzymes in E. coli. Bull Soc Chim Biol (Paris) 1967 Dec 18;49(11):1479–1490. [PubMed] [Google Scholar]
- Kornberg H. L. The role and control of the glyoxylate cycle in Escherichia coli. Biochem J. 1966 Apr;99(1):1–11. doi: 10.1042/bj0990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LIN E. C., LERNER S. A., JORGENSEN S. E. A method for isolating constitutive mutants for carbohydrate-catabolizing enzymes. Biochim Biophys Acta. 1962 Jul 2;60:422–424. doi: 10.1016/0006-3002(62)90423-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- Morrissey A. T., Fraenkel D. G. Chromosomal location of a gene for fructose 6-phosphate kinase in Escherichia coli. J Bacteriol. 1969 Nov;100(2):1108–1109. doi: 10.1128/jb.100.2.1108-1109.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey A. T., Fraenkel D. G. Selection of fructose 6-phosphate kinase mutants in Escherichia coli. Biochem Biophys Res Commun. 1968 Aug 13;32(3):467–473. doi: 10.1016/0006-291x(68)90685-2. [DOI] [PubMed] [Google Scholar]
- Morrissey A. T., Fraenkel D. G. Suppressor of phosphofructokinase mutations of Escherichia coli. J Bacteriol. 1972 Oct;112(1):183–187. doi: 10.1128/jb.112.1.183-187.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. Repression of beta-galactosidase synthesis by glucose in phosphotransferase mutants of Escherichia coli. Repression in the absence of glucose phosphorylation. J Biol Chem. 1969 Nov 10;244(21):5836–5842. [PubMed] [Google Scholar]
- Peyru G., Fraenkel D. G. Genetic mapping of loci for glucose-6-phosphate dehydrogenase, gluconate-6-phosphate dehydrogenase, and gluconate-6-phosphate dehydrase in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1272–1278. doi: 10.1128/jb.95.4.1272-1278.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesnowitz-Horn S., Adelberg E. A. Proflavin-induced mutations in the L-arabinose operon of Escherichia coli. I. Production and genetic analyses of such mutations. J Mol Biol. 1969 Nov 28;46(1):1–15. doi: 10.1016/0022-2836(69)90053-9. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Fraenkel D. G., Lin E. C. The enzymatic lesion of strain MM-6, a pleiotropic carbohydrate-negative mutant of Escherichia coli. Biochem Biophys Res Commun. 1967 Apr 7;27(1):63–67. doi: 10.1016/s0006-291x(67)80040-8. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDERWINKEL E., LIARD P., RAMOS F., WIAME J. M. Genetic control of the regulation of isocitritase and malate synthase in Escherichia coli K 12. Biochem Biophys Res Commun. 1963 Jul 18;12:157–162. doi: 10.1016/0006-291x(63)90254-7. [DOI] [PubMed] [Google Scholar]
- Vanderwinkel E., De Vlieghere M. Physiologie et génétique de l'isocitritase et des malate synthases chez Escherichia coli. Eur J Biochem. 1968 Jun;5(1):81–90. doi: 10.1111/j.1432-1033.1968.tb00340.x. [DOI] [PubMed] [Google Scholar]


