Abstract
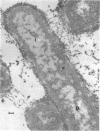
Mutant TH14 of Bacillus megaterium ATCC 19213 is thermosensitive and defective in cell-division septation and spore formation at the restrictive temperature (39 C). As a consequence, the mutant forms multinucleate aseptate filaments and is asporogenic. The mutation does not result in any qualitative compositional changes in extractable membrane proteins. At the restrictive temperature, the mutant membrane has a reduced content of a small molecular weight protein(s). A membrane protein(s) with a molecular weight of nearly 80,000 appears to be partially derepressed in the mutant grown at the restrictive temperature. In addition, numerous unidentified spherical inclusions of fairly uniform size (diameter approximately 100 nm) are present in the cytoplasm at the restrictive temperature. They are especially concentrated at only one pole of each filament. Filamentous growth of the mutant is less sensitive to penicillin than growth in the rod form. Growth in either form is equally sensitive to d-cycloserine at the concentrations used for selection of the mutant. Temperature shift-up experiments suggest that one to two rounds of deoxyribonucleic acid (DNA) replication occur before the phenotypic expression of the mutation occurs. The septations after these replication events can be either two-division septations or a single-division septation plus a subsequent sporulation septation. This conclusion, coupled with previously reported work, supports the hypothesis that the early stages of sporulation represent a modified cell division.
Full text
PDF

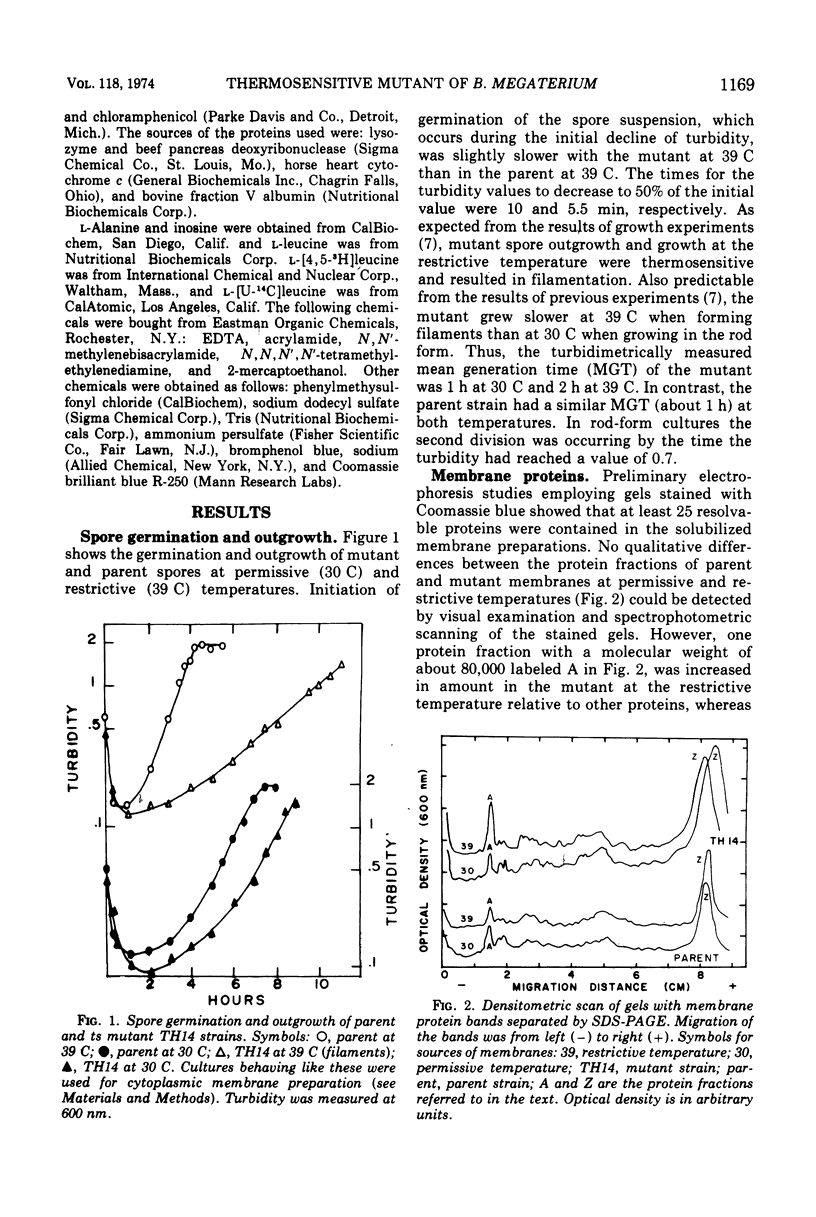


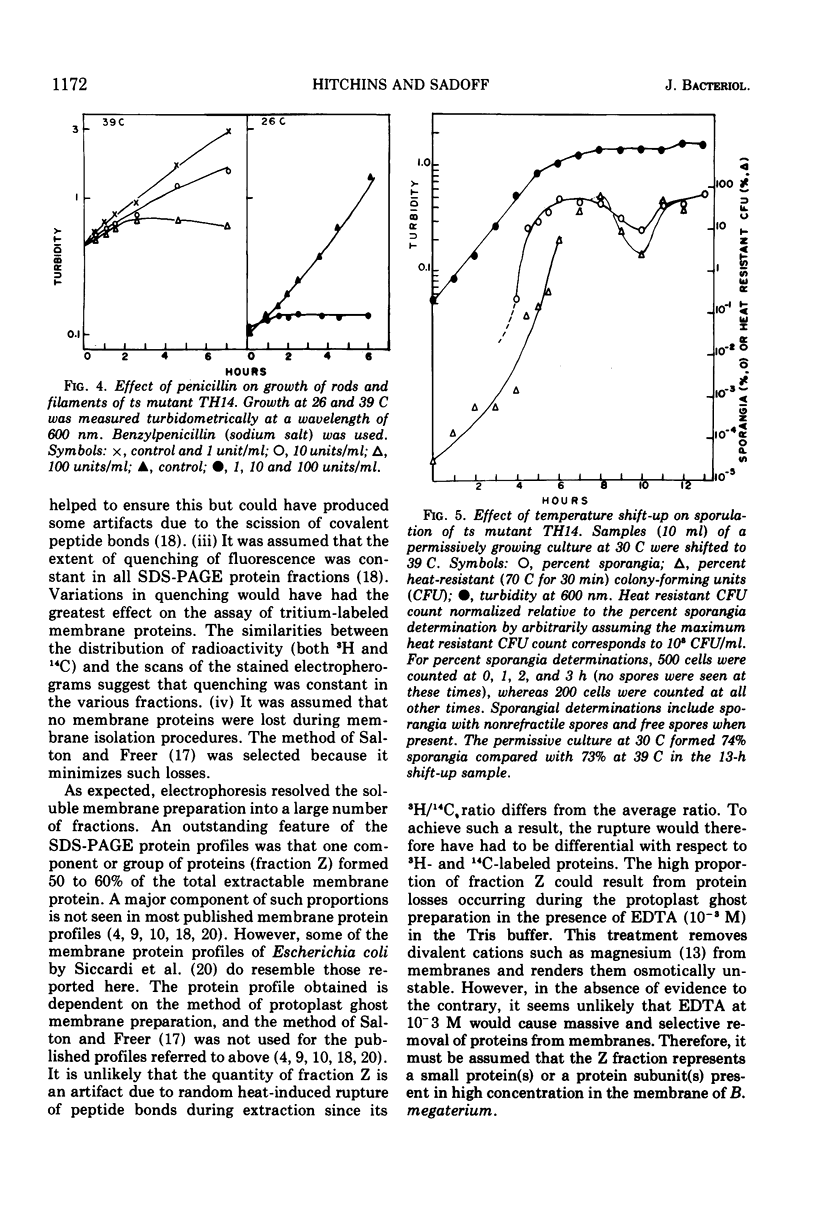
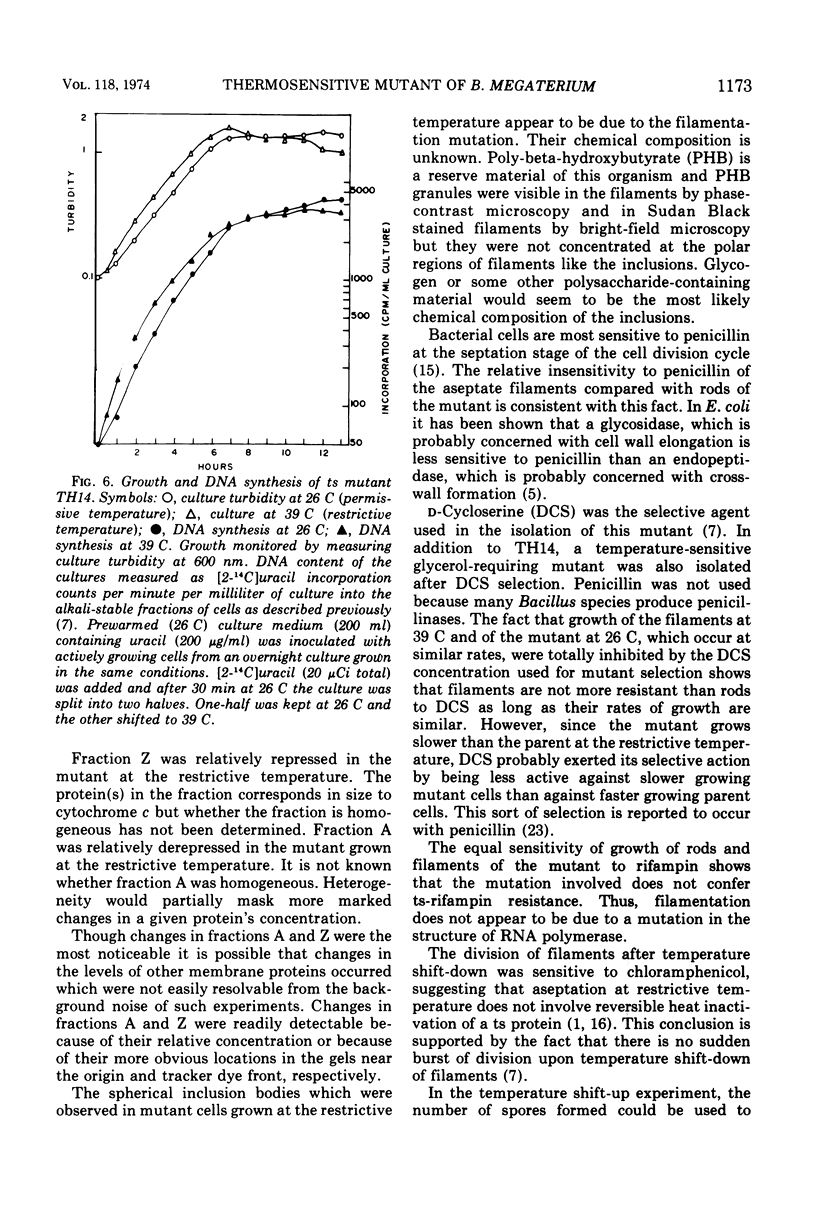


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed N., Rowbury R. J. Temperature-sensitive cell division component in a mutant of Salmonella typhimurium. J Gen Microbiol. 1971 Jul;67(1):107–115. doi: 10.1099/00221287-67-1-107. [DOI] [PubMed] [Google Scholar]
- Grula E. A., Savoy C. F. A detergent-polyacrylamide gel system for electrophoretic resolution of membrane and wall proteins. Biochem Biophys Res Commun. 1971 Apr 16;43(2):325–332. doi: 10.1016/0006-291x(71)90756-x. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Höltje J. V., Schwarz U. Targets of penicillin action in Escherichia coli. Nature. 1972 Feb 25;235(5339):426–429. doi: 10.1038/235426a0. [DOI] [PubMed] [Google Scholar]
- Hitchins A. D., Kahn A. J., Slepecky R. A. Interference contrast and phase contrast microscopy of sporulation and germination of Bacillus megaterium. J Bacteriol. 1968 Nov;96(5):1811–1817. doi: 10.1128/jb.96.5.1811-1817.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchins A. D., Slepecky R. A. Bacterial sporulation as a modified procaryotic cell division. Nature. 1969 Aug 23;223(5208):804–807. doi: 10.1038/223804a0. [DOI] [PubMed] [Google Scholar]
- Inouye M. Internal standards for molecular weight determinations of proteins by polyacrylamide gel electrophoresis. Applications to envelope proteins of Escherichia coli. J Biol Chem. 1971 Aug 10;246(15):4834–4838. [PubMed] [Google Scholar]
- Inouye M., Pardee A. B. Changes of membrane proteins and their relation to deoxyribonucleic acid synthesis and cell division of Escherichia coli. J Biol Chem. 1970 Nov 10;245(21):5813–5819. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lusk J. E., Williams R. J., Kennedy E. P. Magnesium and the growth of Escherichia coli. J Biol Chem. 1968 May 25;243(10):2618–2624. [PubMed] [Google Scholar]
- Mandelstam J., Sterlini J. M., Kay D. Sporulation in Bacillus subtilis. Effect of medium on the form of chromosome replication and on initiation to sporulation in Bacillus subtilis. Biochem J. 1971 Nov;125(2):635–641. doi: 10.1042/bj1250635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathison G. E. Kinetics of death induced by penicillin and chloramphenicol in synchronous cultures of Escherichia coli. Nature. 1968 Jul 27;219(5152):405–407. doi: 10.1038/219405a0. [DOI] [PubMed] [Google Scholar]
- Nagai K., Kaneko H., Tamura G. Thermosensitive mutant of Escherichia coli requiring new protein synthesis to recover cellular division ability. Biochem Biophys Res Commun. 1971 Feb 19;42(4):669–675. doi: 10.1016/0006-291x(71)90540-7. [DOI] [PubMed] [Google Scholar]
- SLEPECKY R., FOSTER J. W. Alterations in metal content of spores of Bacillus megaterium and the effect on some spore properties. J Bacteriol. 1959 Jul;78(1):117–123. doi: 10.1128/jb.78.1.117-123.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R., Freer J. H. Composition of the membranes isolated from several Gram-positive bacteria. Biochim Biophys Acta. 1965 Oct 18;107(3):531–538. doi: 10.1016/0304-4165(65)90197-2. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M., Siccardi A. G., Hirota Y., Jacob F. On the process of cellular division in Escherichia coli. Membrane prtein alterations associated with mutations affecting the initiation of DNA synthesis. J Mol Biol. 1970 Aug 28;52(1):75–89. doi: 10.1016/0022-2836(70)90178-6. [DOI] [PubMed] [Google Scholar]
- Siccardi A. G., Shapiro B. M. On the process of cellular division in Escherichia coli. IV. Altered protein composition and turnover of the membranes of thermosensitive mutants defective in chromosomal replication. J Mol Biol. 1971 Mar 28;56(3):475–490. doi: 10.1016/0022-2836(71)90395-0. [DOI] [PubMed] [Google Scholar]
- Szulmajster J., Bonamy C., Laporte J. Isolation and properties of a temperature-sensitive sporulation mutant of Bacillus subtilis. J Bacteriol. 1970 Mar;101(3):1027–1037. doi: 10.1128/jb.101.3.1027-1037.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- von Meyenburg Kaspar Transport-limited growth rates in a mutant of Escherichia coli. J Bacteriol. 1971 Sep;107(3):878–888. doi: 10.1128/jb.107.3.878-888.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]