Abstract
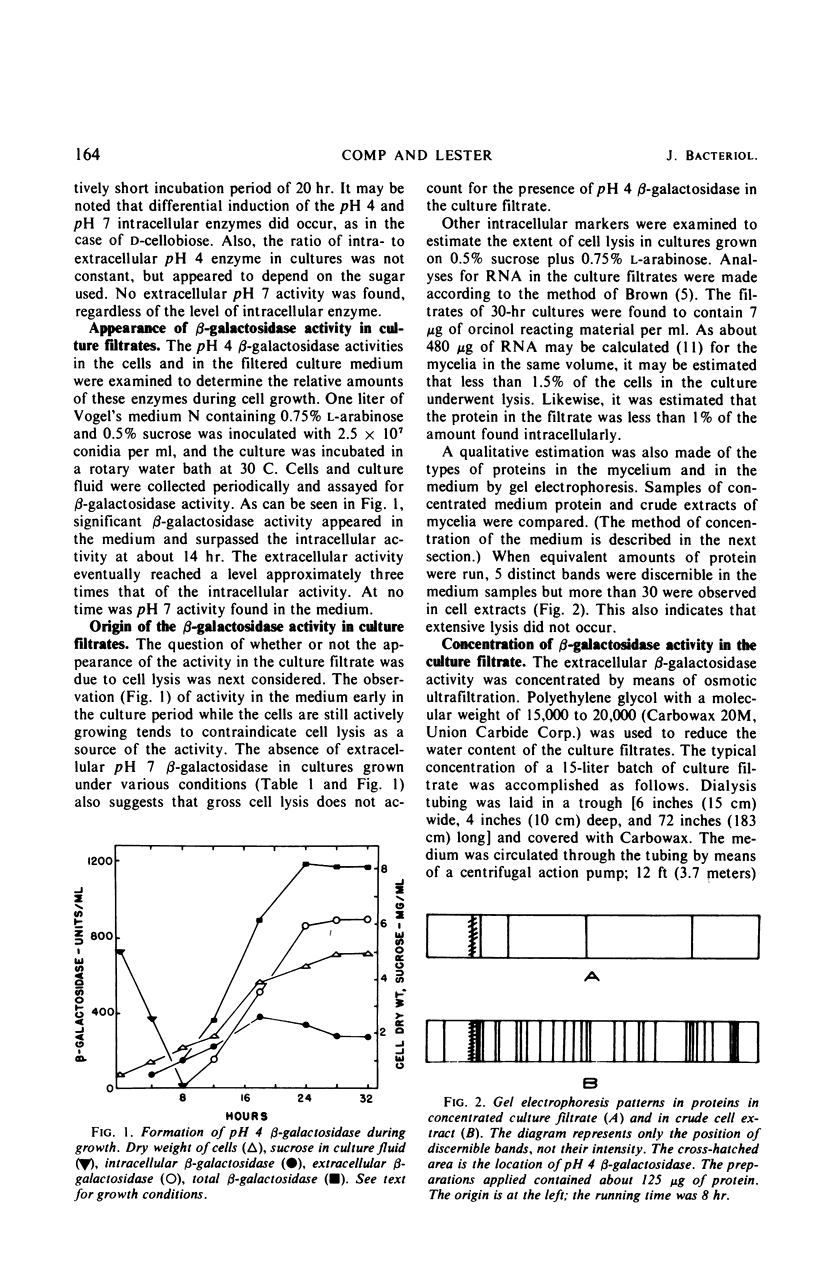
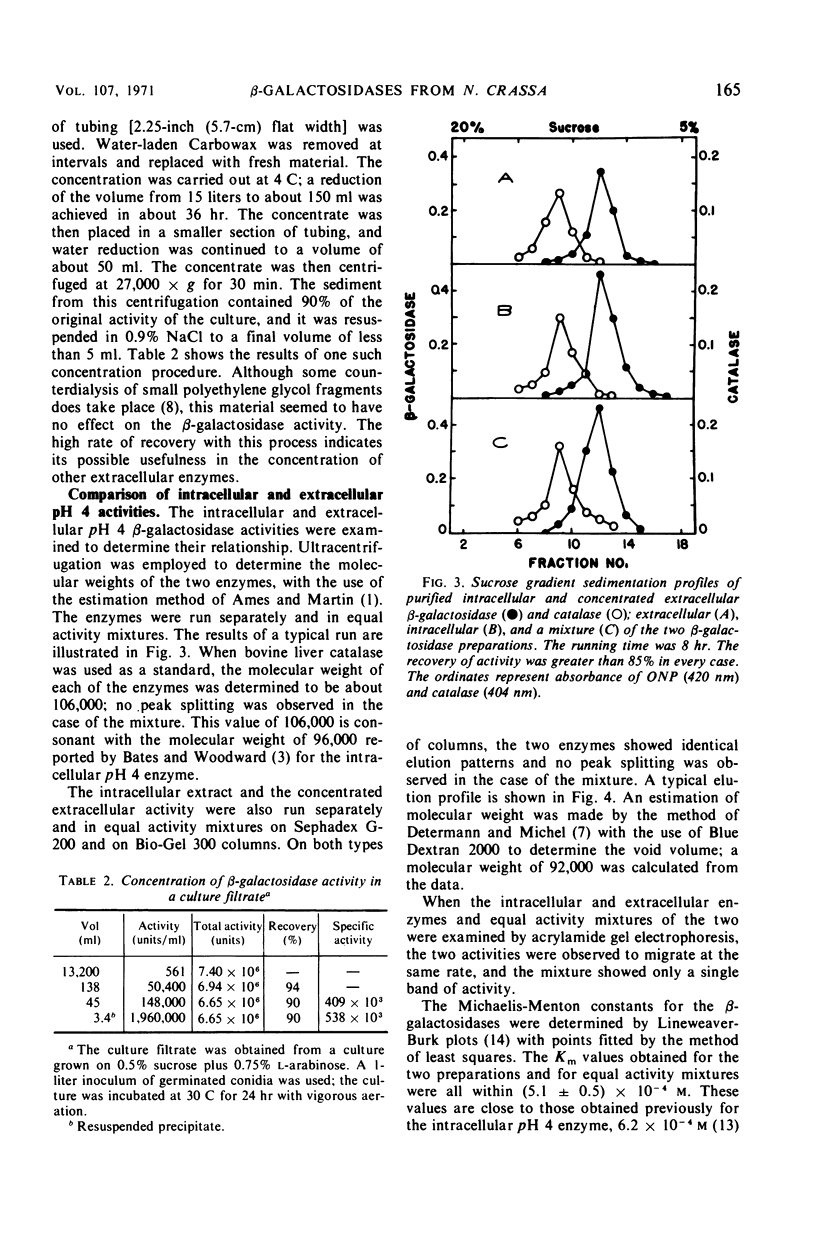
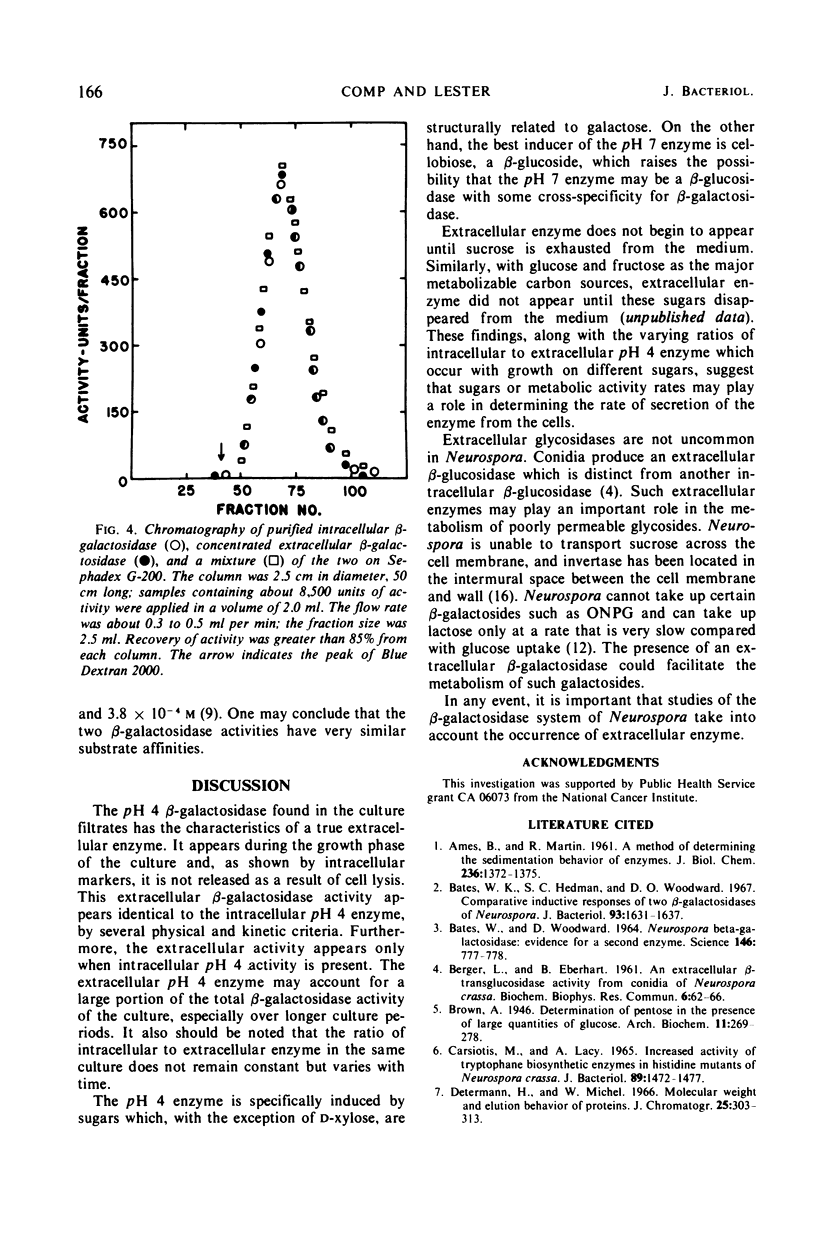
An extracellular β-galactosidase with an activity optimum at about pH 4 was found to occur in the filtrates of Neurospora crassa strain 74A when this mold was grown on certain sugars. This activity accounted for a substantial portion of the β-galactosidase activity in the culture. The β-galactosidase in the medium appeared to be a secreted, extracellular enzyme, not a product of cell lysis. The extracellular activity was found to have physical and kinetic properties similar to those of an intracellular β-galactosidase previously found in Neurospora. Some conditions for the production and concentration of the enzyme are described.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATES W. K., WOODWARD D. O. NEUROSPORA BETA-GALACTOSIDASE: EVIDENCE FOR A SECOND ENZYME. Science. 1964 Nov 6;146(3645):777–778. doi: 10.1126/science.146.3645.777. [DOI] [PubMed] [Google Scholar]
- BERGER L. S., EBERHART B. M. Extracellular beta-transglucosidase activity from conidia of Neurospora crassa. Biochem Biophys Res Commun. 1961 Oct 23;6:62–66. doi: 10.1016/0006-291x(61)90186-3. [DOI] [PubMed] [Google Scholar]
- Bates W. K., Hedman S. C., Woodward D. O. Comparative inductive responses of two beta-galactosidases of Neurospora. J Bacteriol. 1967 May;93(5):1631–1637. doi: 10.1128/jb.93.5.1631-1637.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARSIOTIS M., LACY A. M. INCREASED ACTIVITY OF TRYPTOPHAN BIOSYNTHETIC ENZYMES IN HISTIDINE MUTANTS OF NEUROSPORA CRASSA. J Bacteriol. 1965 Jun;89:1472–1477. doi: 10.1128/jb.89.6.1472-1477.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWE A. F., GROOM T., CARTER R. G. USE OF POLYETHYLENE GLYCOL IN THE CONCENTRATION OF PROTEIN SOLUTIONS. Anal Biochem. 1964 Dec;9:443–453. doi: 10.1016/0003-2697(64)90205-2. [DOI] [PubMed] [Google Scholar]
- LANDMAN O. E. Neurospora lactase. II. Enzyme formation in the standard strain. Arch Biochem Biophys. 1954 Sep;52(1):93–109. doi: 10.1016/0003-9861(54)90092-2. [DOI] [PubMed] [Google Scholar]
- LESTER G., AZZENA D., HECHTER O. Permeability and metabolism of lactose in Neurospora crassa. J Bacteriol. 1962 Aug;84:217–227. doi: 10.1128/jb.84.2.217-227.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER G. Some aspects of tryptophan synthetase formation in Neurospora crassa. J Bacteriol. 1961 Jun;81:964–973. doi: 10.1128/jb.81.6.964-973.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lester G. Inhibition of Growth, Synthesis, and Permeability in Neurospora crassa by Phenethyl Alcohol. J Bacteriol. 1965 Jul;90(1):29–37. doi: 10.1128/jb.90.1.29-37.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]


