Abstract
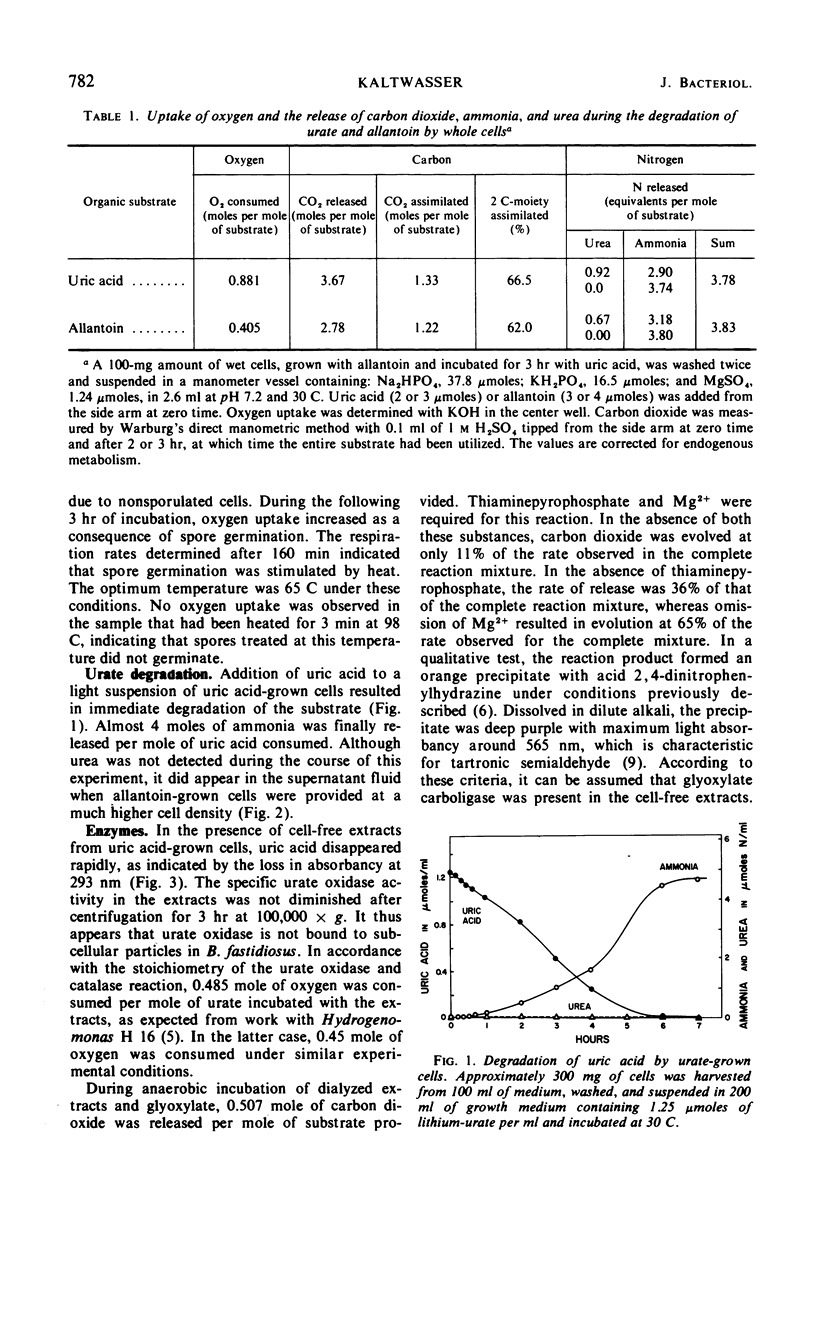
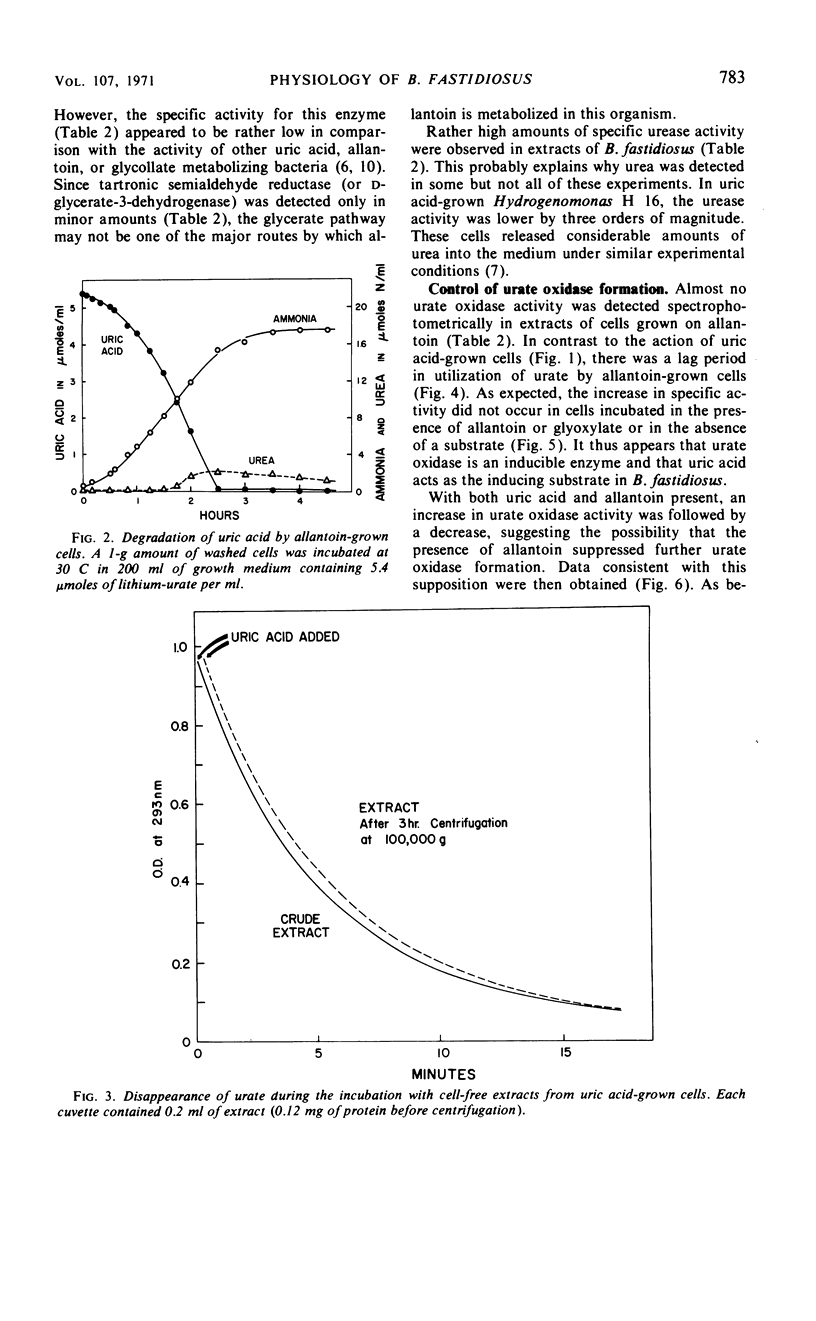
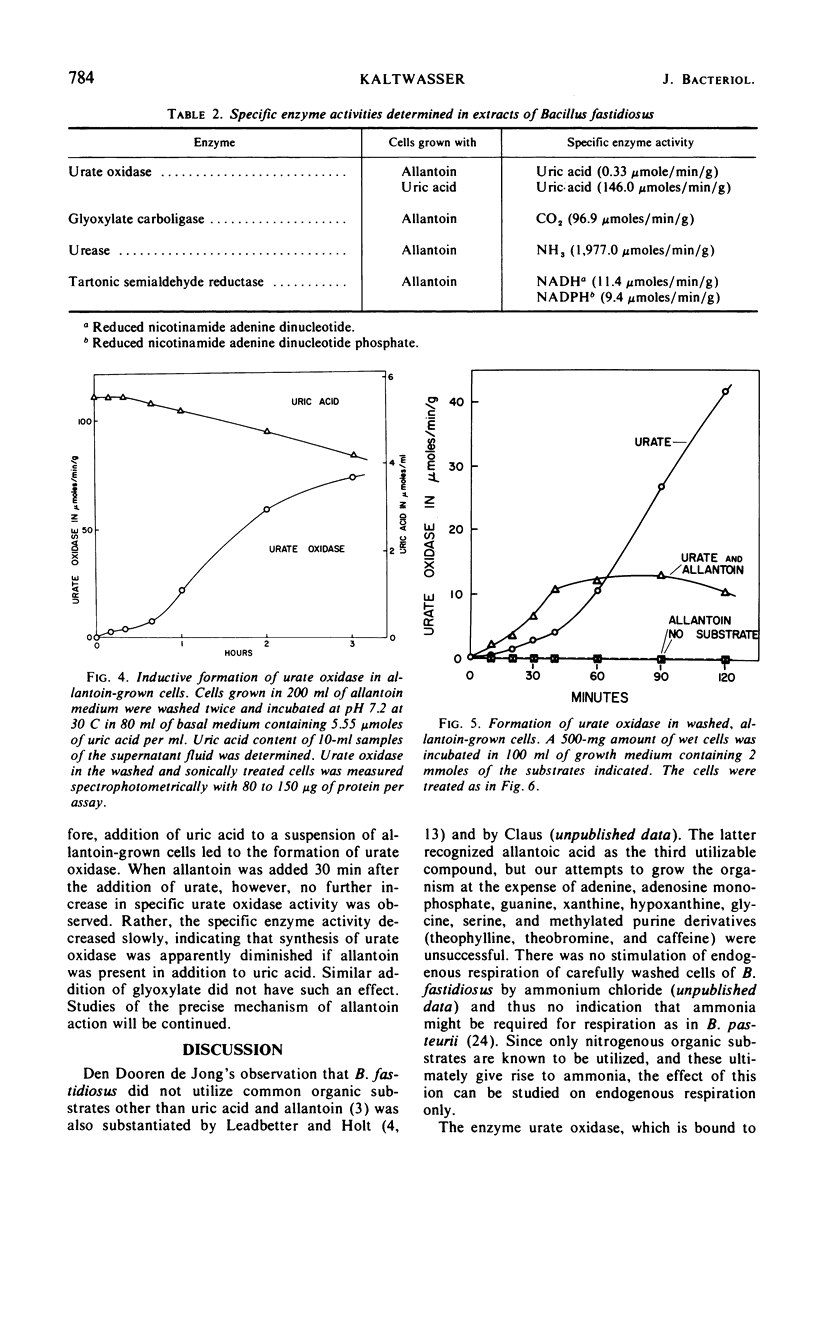
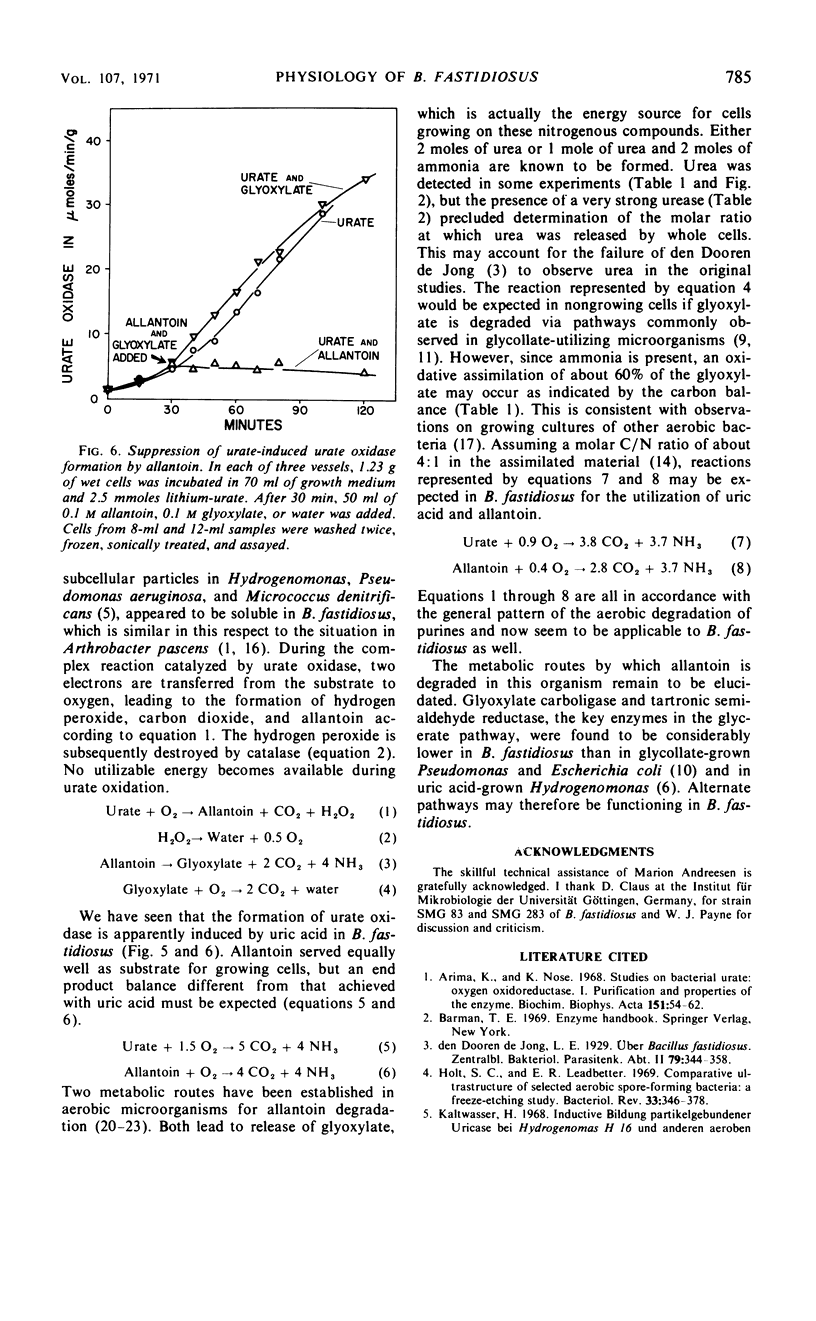
Bacillus fastidiosus was grown in a minimal medium that contained uric acid or allantoin, aerated by vigorous stirring. A constant, optimum pH of 7.4 was maintained by controlled addition of sulfuric acid. Washed cells converted both urate and allantoin into carbon dioxide and ammonia, simultaneously assimilating part of the available carbon and nitrogen. Urate oxidase (formerly called uricase) was present in extracts from urate-grown but not allantoin-grown cells. The formation of urate oxidase was apparently induced by urate. Urea was detected as an intermediate in some but not all of these experiments. However, the high urease activity observed in cell-free extracts may have prevented accumulation of urea in many of the experiments. The presence of glyoxylate carboligase and tartronic semialdehyde reductase activities indicates that the glycerate pathway may be involved in urate and allantoin catabolism in this organism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima K., Nose K. Studies on bacterial urate:oxygen oxidoreductase. I. Purification and properties of the enzyme. Biochim Biophys Acta. 1968 Jan 8;151(1):54–62. doi: 10.1016/0005-2744(68)90160-5. [DOI] [PubMed] [Google Scholar]
- BARKULIS S. S., KRAKOW G. Conversion of glyoxylate to hydroxypyruvate by extracts of Escherichia coli. Biochim Biophys Acta. 1956 Sep;21(3):593–594. doi: 10.1016/0006-3002(56)90208-6. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969 Jun;33(2):346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., GOTTO A. M. The metabolism of C2 compounds in micro-organisms. 6. Synthesis of cell constituents from glycollate by Pseudomonas sp. Biochem J. 1961 Jan;78:69–82. doi: 10.1042/bj0780069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., MORRIS J. G. THE UTILIZATION OF GLYCOLLATE BY MICROCOCCUS DENITRIFICANS: THE BETA-HYDROXYASPARTATE PATHWAY. Biochem J. 1965 Jun;95:577–586. doi: 10.1042/bj0950577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., SADLER J. R. The metabolism of C2-compounds in micro-organisms. VIII. A dicarboxylic acid cycle as a route for the oxidation of glycollate by Escherichia coli. Biochem J. 1961 Dec;81:503–513. doi: 10.1042/bj0810503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltwasser H. Harnsäureabbau und Biosynthese der Enzyme Uricase, Glyoxylatcarboligase und Urease bei Hydrogenomonas H 16. I. Bildung von Glyoxylatcarbioligase und D-Glycerat-3-Dehydrogenase. Arch Mikrobiol. 1968;64(1):71–84. [PubMed] [Google Scholar]
- Kaltwasser H. Harnsäureabbau und Biosynthese der Enzyme uricase, glyoxylatcarboligase und Urease bei Hydrogenomonas H 16. II. Einfluss von Harnsäure, Fructose und Stickstoffmangel. Arch Mikrobiol. 1969;65(3):288–302. [PubMed] [Google Scholar]
- Kaltwasser H. Induktive Bildung partikelgebundener Uricase bei Hydrogenomonas H16 und anderen aeroben Bakterien. Arch Mikrobiol. 1968;60(2):160–171. [PubMed] [Google Scholar]
- Kaltwasser H., Schlegel H. G. NADH-Dependent coupled enzyme assay for urease and other ammonia-producing systems. Anal Biochem. 1966 Jul;16(1):132–138. doi: 10.1016/0003-2697(66)90088-1. [DOI] [PubMed] [Google Scholar]
- Mahler J. L. A new bacterial uricase for uric acid determination. Anal Biochem. 1970 Nov;38(1):65–84. doi: 10.1016/0003-2697(70)90156-9. [DOI] [PubMed] [Google Scholar]
- Nose K., Arima K. Studies on bacterial urate:oxygen oxidoreductase. II. Observations concerning the properties and components of the active site. Biochim Biophys Acta. 1968 Jan 8;151(1):63–69. doi: 10.1016/0005-2744(68)90161-7. [DOI] [PubMed] [Google Scholar]
- Payne W. J. Energy yields and growth of heterotrophs. Annu Rev Microbiol. 1970;24:17–52. doi: 10.1146/annurev.mi.24.100170.000313. [DOI] [PubMed] [Google Scholar]
- SCHLEGEL H. G., KALTWASSER H., GOTTSCHALK G. [A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies]. Arch Mikrobiol. 1961;38:209–222. [PubMed] [Google Scholar]
- SCHMIDT K., LIAAENJENSEN S., SCHLEGEL H. G. DIE CAROTINOIDE DER THIORHODACEAE. I. OKENON ALS HAUPTEAROTINOID VON CHROMATIUM OKENII PERTY. Arch Mikrobiol. 1963 Aug 1;46:117–126. [PubMed] [Google Scholar]
- Trijbels F., Vogels G. D. Allantoate and ureidoglycolate degradation by Pseudomonas aeruginosa. Biochim Biophys Acta. 1967 Jan 11;132(1):115–126. doi: 10.1016/0005-2744(67)90197-0. [DOI] [PubMed] [Google Scholar]
- Trijbels F., Vogels G. D. Allantoicase and ureidoglycolase in Pseudomonas and Penicillium species. Biochim Biophys Acta. 1966 May 5;118(2):387–395. doi: 10.1016/s0926-6593(66)80047-4. [DOI] [PubMed] [Google Scholar]
- Trijbels F., Vogels G. D. Degradation of allantoin by Pseudomonas acidovorans. Biochim Biophys Acta. 1966 Feb 14;113(2):292–301. doi: 10.1016/s0926-6593(66)80068-1. [DOI] [PubMed] [Google Scholar]
- Vogels G. D. Stereospecificity in the allantoin metabolism. Antonie Van Leeuwenhoek. 1969;35(2):236–238. doi: 10.1007/BF02219137. [DOI] [PubMed] [Google Scholar]
- WILEY W. R., STOKES J. L. Requirement of an alkaline pH and ammonia for substrate oxidation by Bacillus pasteurii. J Bacteriol. 1962 Oct;84:730–734. doi: 10.1128/jb.84.4.730-734.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]


