Abstract
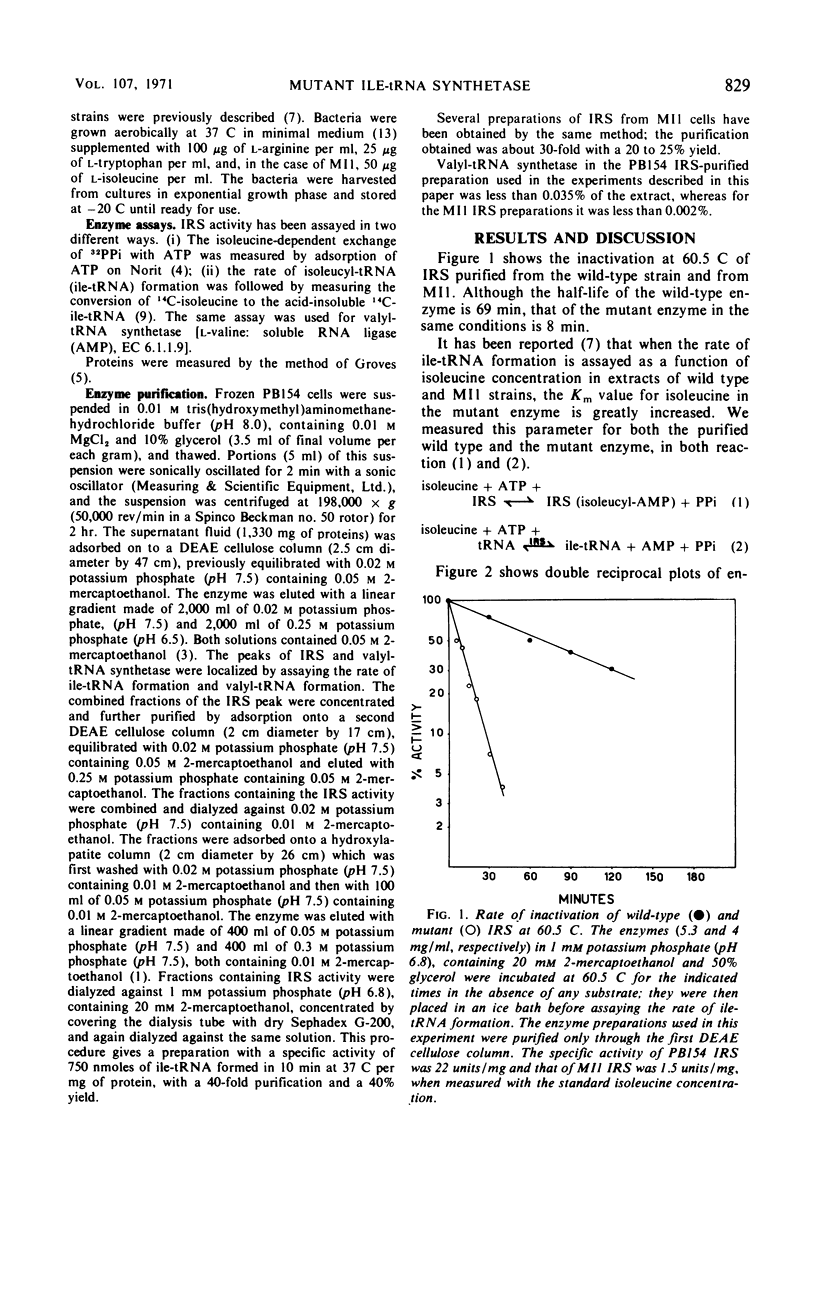
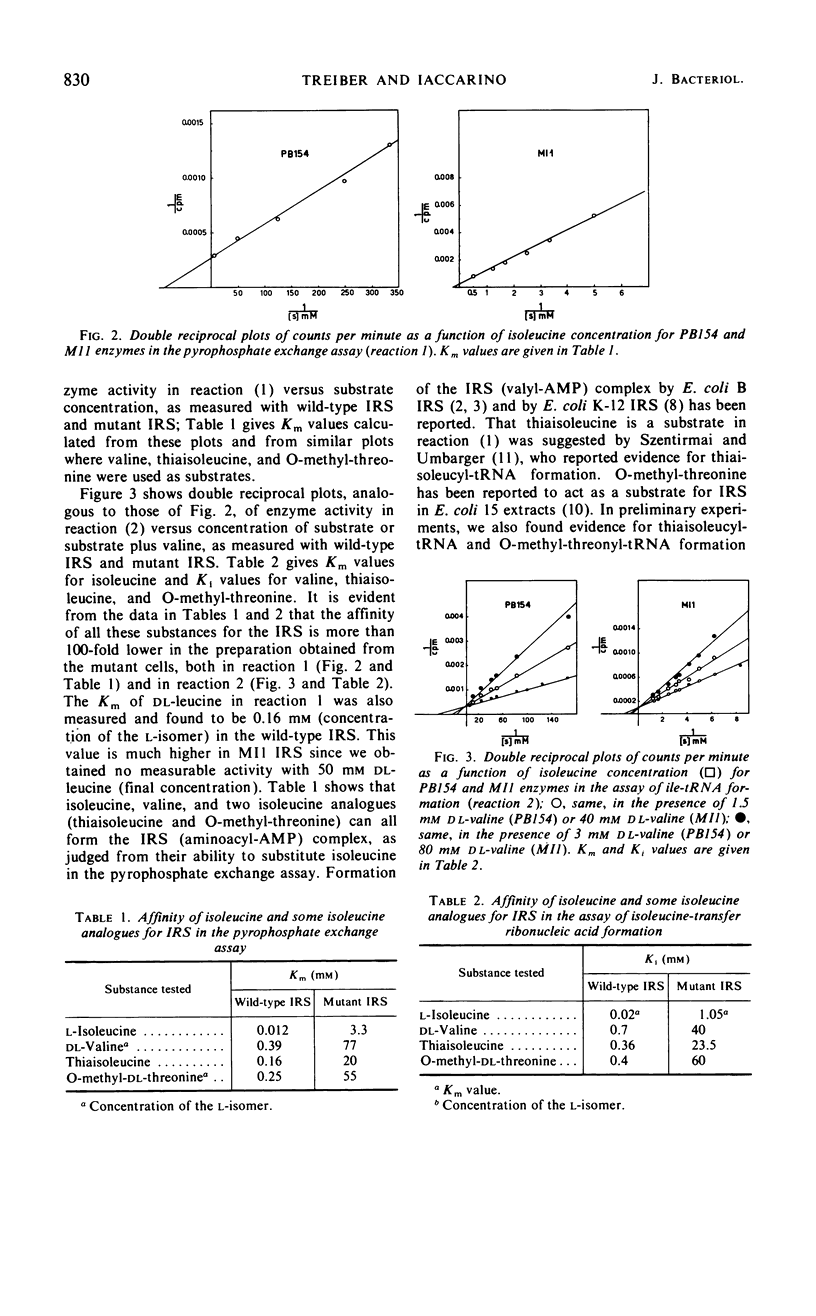
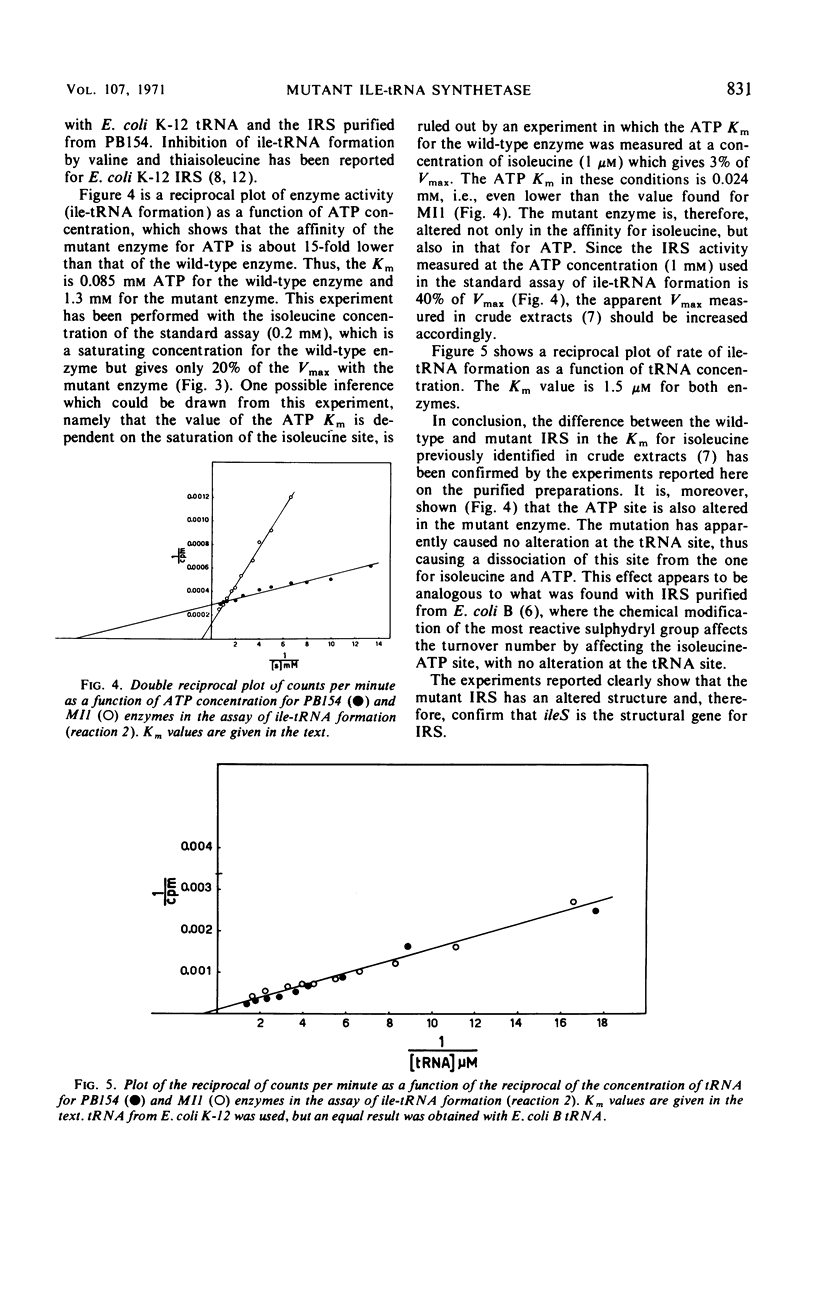
Isoleucyl-transfer ribonucleic acid (tRNA) synthetase [l-isoleucine: soluble RNA ligase (adenosine monophosphate), EC 6.1.1.5; IRS] was partially purified from Escherichia coli K-12 and from an ileS mutant that appears to be altered in IRS. The half-life of wild-type IRS, incubated at 60.5 C, is 69 min, whereas that of mutant IRS is 8 min. Mutant IRS shows about a 100-fold lower affinity than wild-type IRS for isoleucine, dl-valine, thiaisoleucine, and O-methyl-dl-threonine, both in the pyrophosphate exchange assay and in the assay of isoleucyl-tRNA formation. The affinity of the mutant enzyme for adenosine triphosphate in the assay of isoleucyl-tRNA formation is 15-fold lower than that of the wild-type enzyme. The affinity of mutant IRS for tRNA is not changed as compared with wild-type IRS. These data show that mutant IRS has an altered structure and clearly confirm that ileS is the structural gene for IRS.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin A. N., Berg P. Purification and properties of isoleucyl ribonucleic acid synthetase from Escherichia coli. J Biol Chem. 1966 Feb 25;241(4):831–838. [PubMed] [Google Scholar]
- Baldwin A. N., Berg P. Transfer ribonucleic acid-induced hydrolysis of valyladenylate bound to isoleucyl ribonucleic acid synthetase. J Biol Chem. 1966 Feb 25;241(4):839–845. [PubMed] [Google Scholar]
- Groves W. E., Davis F. C., Jr, Sells B. H. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal Biochem. 1968 Feb;22(2):195–210. doi: 10.1016/0003-2697(68)90307-2. [DOI] [PubMed] [Google Scholar]
- Iaccarino M., Berg P. Isoleucine auxotrophy as a consequence of a mutationally altered isoleucyl-transfer ribonucleic acid synthetase. J Bacteriol. 1971 Feb;105(2):527–537. doi: 10.1128/jb.105.2.527-537.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino M., Berg P. Requirement of sulfhydryl groups for the catalytic and tRNA recognition functions of isoleucyl-tRNA synthetase. J Mol Biol. 1969 Jun 14;42(2):151–169. doi: 10.1016/0022-2836(69)90036-9. [DOI] [PubMed] [Google Scholar]
- Kondo M., Woese C. R. Specificity of aminoacyl transfer ribonucleic acid synthetases from Escherichia coli K12. Biochemistry. 1969 Oct;8(10):4177–4182. doi: 10.1021/bi00838a040. [DOI] [PubMed] [Google Scholar]
- Smulson M. E., Rabinovitz M., Breitman T. R. O-methylthreonine inhibition of growth and of threonine deaminase in Escherichia coli. J Bacteriol. 1967 Dec;94(6):1890–1895. doi: 10.1128/jb.94.6.1890-1895.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai A., Szentirmai M., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XV. Biochemical properties of mutants resistant to thiaisoleucine. J Bacteriol. 1968 May;95(5):1672–1679. doi: 10.1128/jb.95.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai A., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XIV. Effect of thiaisoleucine. J Bacteriol. 1968 May;95(5):1666–1671. doi: 10.1128/jb.95.5.1666-1671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Williams L. S., Neidhardt F. C. Synthesis and inactivation of aminoacyl-transfer RNA synthetases during growth of Escherichia coli. J Mol Biol. 1969 Aug 14;43(3):529–550. doi: 10.1016/0022-2836(69)90357-x. [DOI] [PubMed] [Google Scholar]


