Abstract
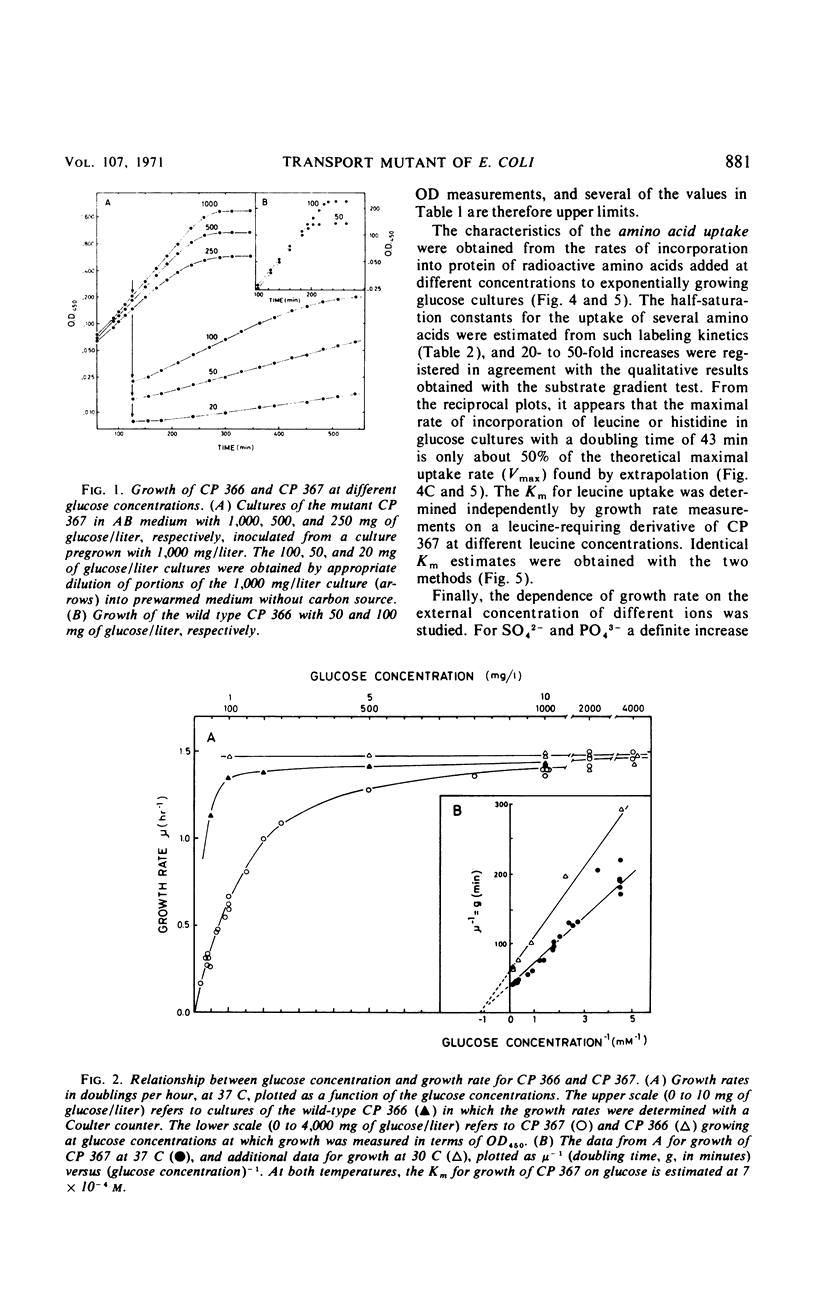
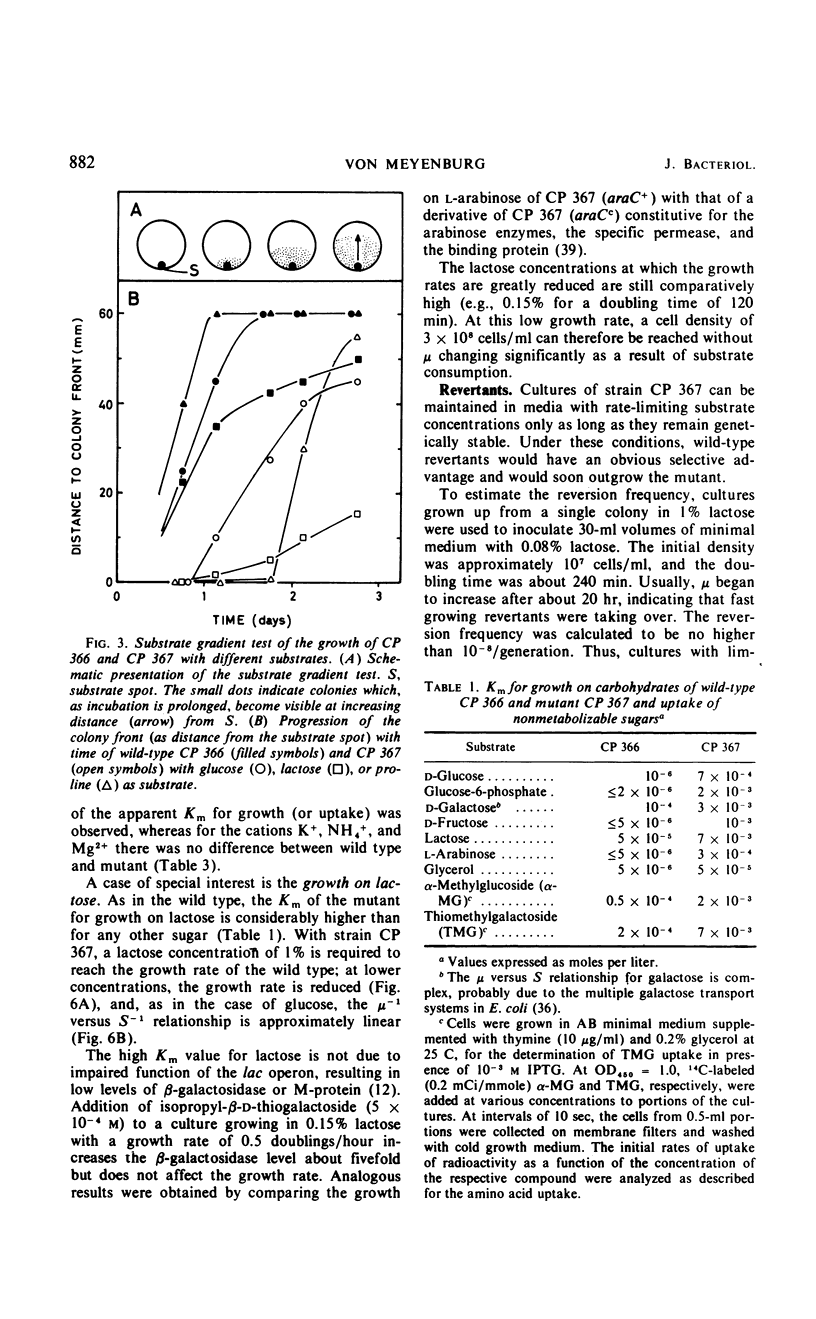
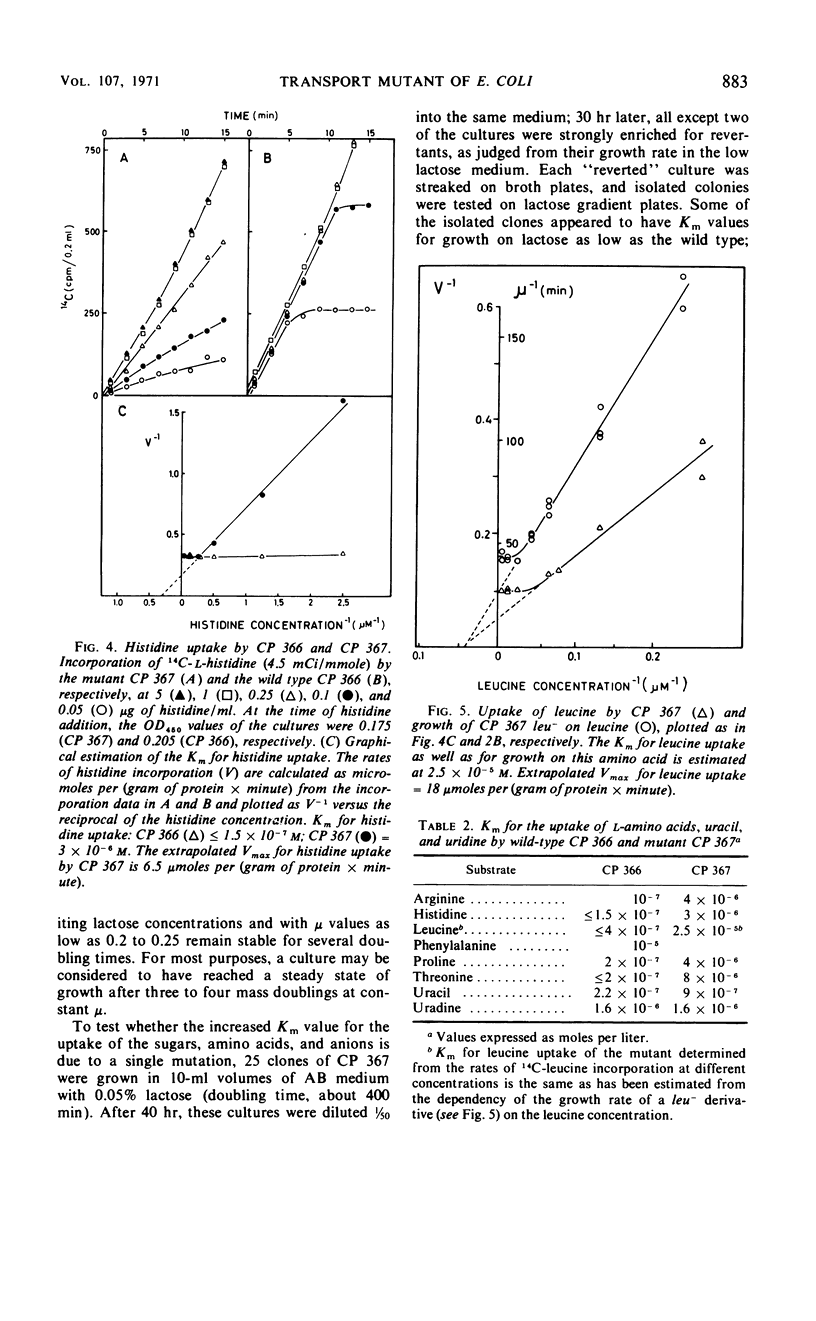
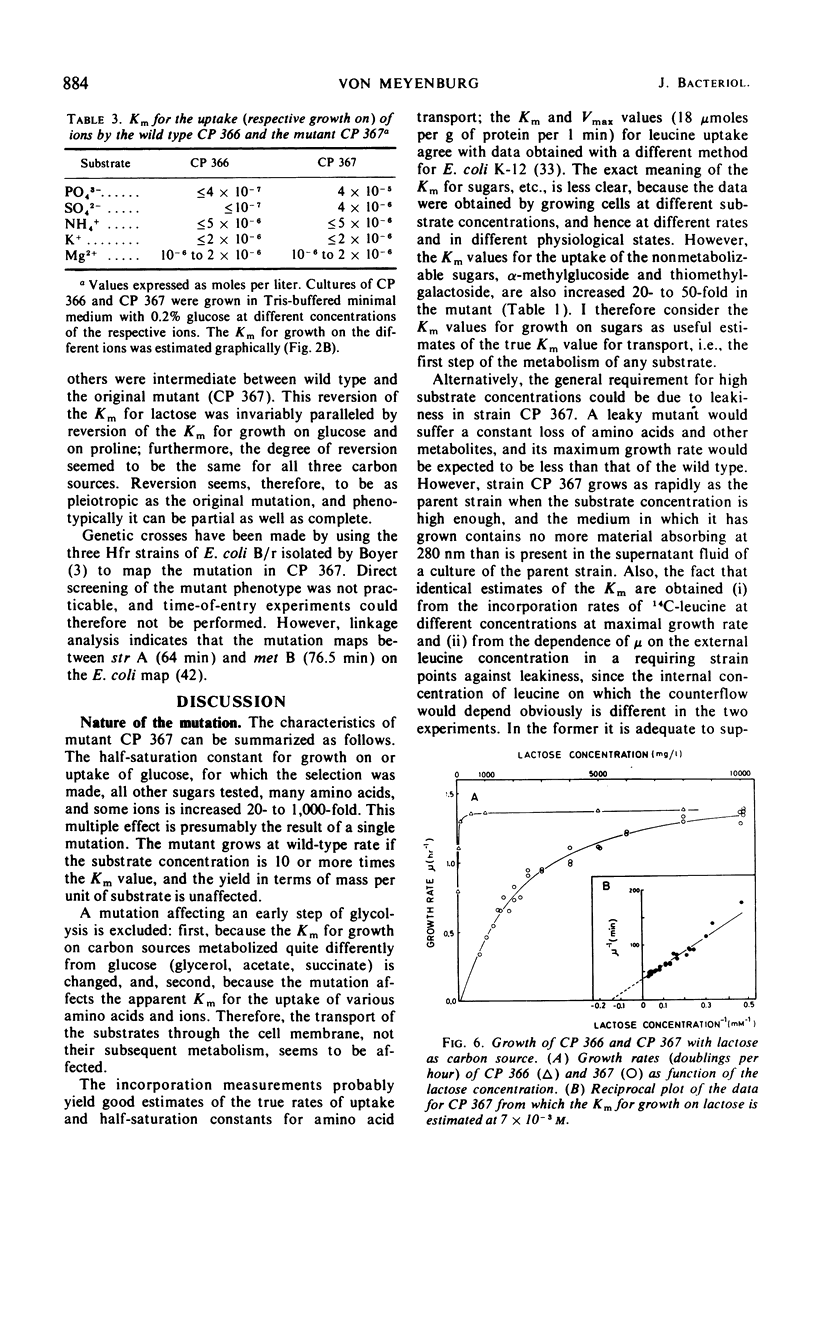
Mutants of Escherichia coli B/r have been selected which require increased nutrient concentrations for half-maximal growth rate. This half-saturation constant (Km) for growth on glucose is 10−6 and 7 × 10−4m for the wild type (CP 366) and the mutant (CP 367), respectively. Similarly, the Km is increased for growth on many other carbohydrates (20- to 500-fold), for the anions PO43− and SO42− (ca. 100-fold), and for the uptake of several amino acids (20- to 50-fold). At sufficiently high concentrations of the nutrients, mutant and wild type grow equally fast. The yield in terms of cell mass per milligram of substrate is unaffected by the mutation. The phenotype of the parent is reestablished in what appears to be the reversion of a single mutation (kmt) which maps between strA and metB. The pleiotropic decrease of the affinities for transport of the various nutrients seems to be the result of a modification of the cell envelope which weakens the attachment of the various specific binding proteins to the periplasmic membrane. Since the mutant Km values are increased considerably, high cell densities can be reached in batch cultures at growth-rate-limiting substrate concentrations (107 to 108 cells/ml). This allows chemical analysis of the cell composition; the application of the mutant to studies of bacterial physiology as function of growth rate is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku Y. Transport of sugars and amino acids in bacteria. I. Purification and specificity of the galactose- and leucine-binding proteins. J Biol Chem. 1968 Jun 10;243(11):3116–3122. [PubMed] [Google Scholar]
- Beck C., von Meyenburg H. K. Enzyme pattern and aerobic growth of Saccharomyces cerevisiae under various degrees of glucose limitation. J Bacteriol. 1968 Aug;96(2):479–486. doi: 10.1128/jb.96.2.479-486.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. Conjugation in Escherichia coli. J Bacteriol. 1966 May;91(5):1767–1772. doi: 10.1128/jb.91.5.1767-1772.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá-Olmedo E., Hanawalt P. C., Guerola N. Mutagenesis of the replication point by nitrosoguanidine: map and pattern of replication of the Escherichia coli chromosome. J Mol Biol. 1968 May 14;33(3):705–719. doi: 10.1016/0022-2836(68)90315-x. [DOI] [PubMed] [Google Scholar]
- ECKER R. E., SCHAECHTER M. RIBOSOME CONTENT AND THE RATE OF GROWTH OF SALMONELLA TYPHIMURIUM. Biochim Biophys Acta. 1963 Oct 15;76:275–279. [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Fleck A., Begg D. The estimation of ribonucleic acid using ultraviolet absorption measurements. Biochim Biophys Acta. 1965 Nov 8;108(3):333–339. doi: 10.1016/0005-2787(65)90025-0. [DOI] [PubMed] [Google Scholar]
- Forchhammer J., Lindahl L. Growth rate of polypeptide chains as a function of the cell growth rate in a mutant of Escherichia coli 15. J Mol Biol. 1971 Feb 14;55(3):563–568. doi: 10.1016/0022-2836(71)90337-8. [DOI] [PubMed] [Google Scholar]
- Fox C. F. A lipid requirement for induction of lactose transport in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):850–855. doi: 10.1073/pnas.63.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Kennedy E. P. Specific labeling and partial purification of the M protein, a component of the beta-galactoside transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Sep;54(3):891–899. doi: 10.1073/pnas.54.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., KAUFMAN H. Selecting bacterial mutants by the penicillin method. Science. 1960 Feb 26;131(3400):604–605. doi: 10.1126/science.131.3400.604. [DOI] [PubMed] [Google Scholar]
- HELMSTETTER C. E., CUMMINGS D. J. AN IMPROVED METHOD FOR THE SELECTION OF BACTERIAL CELLS AT DIVISION. Biochim Biophys Acta. 1964 Mar 16;82:608–610. doi: 10.1016/0304-4165(64)90453-2. [DOI] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Deppe C. S. In vivo assay of protein synthesizing capacity of Escherichia coli from slowly growing chemostat cultures. J Mol Biol. 1971 Feb 14;55(3):549–562. doi: 10.1016/0022-2836(71)90336-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindahl L., Forchhammer J. Evidence for reduced breakdown of messenger RNA during blocked transcription or translation in Escherichia coli. J Mol Biol. 1969 Aug 14;43(3):593–606. doi: 10.1016/0022-2836(69)90361-1. [DOI] [PubMed] [Google Scholar]
- Medveczky N., Rosenberg H. The binding and release of phosphate by a protein isolated from Escherichia coli. Biochim Biophys Acta. 1969 Nov 18;192(2):369–371. doi: 10.1016/0304-4165(69)90382-1. [DOI] [PubMed] [Google Scholar]
- NOVICK A., SZILARD L. Experiments with the Chemostat on spontaneous mutations of bacteria. Proc Natl Acad Sci U S A. 1950 Dec;36(12):708–719. doi: 10.1073/pnas.36.12.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Nichoalds G. E., Oxender D. L. Cellular localization of leucine-binding protein from Escherichia coli. Science. 1968 Jul 12;161(3837):182–183. doi: 10.1126/science.161.3837.182. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Normark S. Mutation in Escherichia coli K-12 mediating spherelike envelopes and changes tolerance to ultraviolet irradiation and some antibiotics. J Bacteriol. 1969 Jun;98(3):1274–1277. doi: 10.1128/jb.98.3.1274-1277.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. Purification and properties of a sulfate-binding protein from Salmonella typhimurium. J Biol Chem. 1966 Dec 25;241(24):5886–5892. [PubMed] [Google Scholar]
- Pardee A. B., Watanabe K. Location of sulfate-binding protein in Salmonella typhimurium. J Bacteriol. 1968 Oct;96(4):1049–1054. doi: 10.1128/jb.96.4.1049-1054.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penrose W. R., Nichoalds G. E., Piperno J. R., Oxender D. L. Purification and properties of a leucine-binding protein from Escherichia coli. J Biol Chem. 1968 Nov 25;243(22):5921–5928. [PubMed] [Google Scholar]
- Rosset R., Julien J., Monier R. Ribonucleic acid composition of bacteria as a function of growth rate. J Mol Biol. 1966 Jul;18(2):308–320. doi: 10.1016/s0022-2836(66)80248-6. [DOI] [PubMed] [Google Scholar]
- Rotman B., Ganesan A. K., Guzman R. Transport systems for galactose and galactosides in Escherichia coli. II. Substrate and inducer specificities. J Mol Biol. 1968 Sep 14;36(2):247–260. doi: 10.1016/0022-2836(68)90379-3. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- Schleif R. An L-arabinose binding protein and arabinose permeation in Escherichia coli. J Mol Biol. 1969 Nov 28;46(1):185–196. doi: 10.1016/0022-2836(69)90065-5. [DOI] [PubMed] [Google Scholar]
- Schleif R. Control of production of ribosomal protein. J Mol Biol. 1967 Jul 14;27(1):41–55. doi: 10.1016/0022-2836(67)90350-6. [DOI] [PubMed] [Google Scholar]
- Sykes J., Young T. W. Studies on the ribosomes and ribonucleic acids of Aerobacter aerogenes gron at different rates in carbon-limited continuous culture. Biochim Biophys Acta. 1968 Nov 20;169(1):103–116. doi: 10.1016/0005-2787(68)90012-9. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. J., Morse H. G., Morse M. L. Carbohydrate accumulation and metabolism in Escherichia coli: the close linkage and chromosomal location of ctr mutations. J Bacteriol. 1969 May;98(2):605–610. doi: 10.1128/jb.98.2.605-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. J., Morse M. L. Carbohydrate accumulation and metabolism in Escherichia coli. I. Description of pleiotropic mutants. J Mol Biol. 1968 Feb 28;32(1):59–66. doi: 10.1016/0022-2836(68)90145-9. [DOI] [PubMed] [Google Scholar]
- Wilson O. H., Holden J. T. Stimulation of arginine transport in osmotically shocked Escherichia coli W cells by purified arginine-binding protein fractions. J Biol Chem. 1969 May 25;244(10):2743–2749. [PubMed] [Google Scholar]
- Wong P. T., Kashket E. R., Wilson T. H. Energy coupling in the lactose transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Jan;65(1):63–69. doi: 10.1073/pnas.65.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]



